Artículos SCI
2024
2024
Química de Superficies y Catálisis
A review on high-pressure heterogeneous catalytic processes for gas-phase CO2 valorization
Villora-Picó, J.J; González-Arias, J; Pastor-Pérez, L; Odriozola, JA; Reina, TREnvironmental Research, 240 (2024) 117520
Show abstract ▽
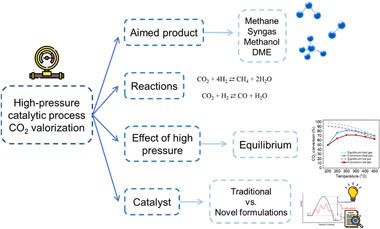
This review discusses the importance of mitigating CO2 emissions by valorizing CO2 through high-pressure catalytic processes. It focuses on various key processes, including CO2 methanation, reverse water-gas shift, methane dry reforming, methanol, and dimethyl ether synthesis, emphasizing pros and cons of high-pressure operation. CO2 methanation, methanol synthesis, and dimethyl ether synthesis reactions are thermodynami-cally favored under high-pressure conditions. However, in the case of methane dry reforming and reverse water -gas shift, applying high pressure, results in decreased selectivity toward desired products and an increase in coke production, which can be detrimental to both the catalyst and the reaction system. Nevertheless, high-pressure utilization proves industrially advantageous for cost reduction when these processes are integrated with Fischer-Tropsch or methanol synthesis units. This review also compiles recent advances in heterogeneous catalysts design for high-pressure applications. By examining the impact of pressure on CO2 valorization and the state of the art, this work contributes to improving scientific understanding and optimizing these processes for sustainable CO2 management, as well as addressing challenges in high-pressure CO2 valorization that are crucial for industrial scaling-up. This includes the development of cost-effective and robust reactor materials and the development of low-cost catalysts that yield improved selectivity and long-term stability under realistic working environments.
Enero, 2024 | DOI: 10.1016/j.envres.2023.117520
Nanotecnología en Superficies y Plasma
Green hydrogen production using doped Fe2O3 foams
Damizia, M; Lloreda-Jurado, PJ; De Filippis, P; de Caprariis, B; Chicardi, E; Sepúlveda, RInternational Journal of Hydrogen Energy, 51 (2024) 834-845
Show abstract ▽
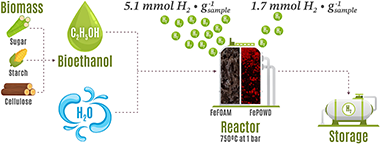
Hydrogen is the ideal energy vector to reduce our fossil-fuels dependency and diminish the climate change consequence. However, current production is still methane based. It is possible to produce hydrogen using bioethanol from the alcoholic fermentation of organic waste by chemical looping processes, but unfortunately current redox systems generate hydrogen with significant traces of CO. In the case of proton exchange membrane fuel cells (PEMFC), hydrogen must be highly purified to produce electricity. Here, high porosity inter-connected Fe2O3 foams doped with 2 wt% Al2O3 were manufactured by the freeze-casting method, obtaining around 5.1 mmol H2$g?1 sample of highly pure hydrogen (<10 ppm of CO) consuming only 3.42 mmol of ethanol on each redox cycles, with no deactivation. This result shows the possibility of using an abundant and inexpensive raw material as the iron oxide to scale-up the direct pure H2 production and facilitates its use in the automotive sector.
Enero, 2024 | DOI: 10.1016/j.ijhydene.2023.09.008
Química de Superficies y Catálisis
Natural hydrogen in the energy transition: Fundamentals, promise, and enigmas
Blay-Roger, R; Bach, W; Bobadilla, LF; Reina, TR; Odriozola, JA; Amils, R; Blay, VRenewable & Sustainable Energy Reviews, 189 (2024) 113888
Show abstract ▽
Beyond its role as an energy vector, a growing number of natural hydrogen sources and reservoirs are being discovered all over the globe, which could represent a clean energy source. Although the hydrogen amounts in reservoirs are uncertain, they could be vast, and they could help decarbonize energy-intensive economic sectors and facilitate the energy transition. Natural hydrogen is mainly produced through a geochemical process known as serpentinization, which involves the reaction of water with low-silica, ferrous minerals. In favorable locations, the hydrogen produced can become trapped by impermeable rocks on its way to the atmosphere, forming a reservoir. The safe exploitation of numerous natural hydrogen reservoirs seems feasible with current technology, and several demonstration plants are being commissioned. Natural hydrogen may show variable composition and require custom separation, purification, storage, and distribution facilities, depending on the location and intended use. By investing in research, in the mid-term, more hydrogen sources could become exploitable and geochemical processes could be artificially stimulated in new locations. In the long term, it may be possible to leverage or engineer the interplay between microorganisms and geological substrates to obtain hydrogen and other chemicals in a sustainable manner.
Enero, 2024 | DOI: 10.1016/j.rser.2023.113888
2023
2023
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
Photoreforming of glycerol to produce hydrogen from natural water in a compound parabolic collector solar photoreactor
Villachica-Llamosas, JG; Sowik, J; Ruiz-Aguirre, A; Colón, G; Peral, J; Malato, SJournal of Environmental Chemical Engineering, 11 (2023) 111216
Show abstract ▽
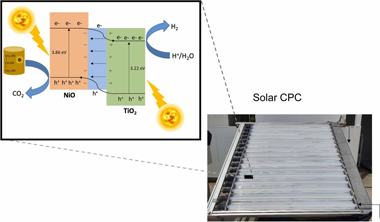
To improve TiO2 for H2 generation, one strategy for the separation of photogenerated charges is the formation of heterostructures with other materials. In particular, NiO is a photocatalyst known for its good stability and low cost. However, no studies at pilot scale using solar energy have been described. Consequently, an evaluation of a physical NiO:TiO2 mixture at pilot scale (25 L) with natural irradiation (2.10 m2 of sun-exposed surface) and with simultaneous glycerol photoreforming was explored. NiO:TiO2 50 mg & sdot;L- 1 resulted in the highest hydrogen production, showing an STH = 1.44%, considering only the UV fraction of the solar irradiation. H2 and CO2 production were analysed by on-line GC; Glycerol, dissolved organic carbon, carboxylic acids and nickel leaching were also evaluated. The NiO:TiO2 mixtures rendered a systematically lower H2 production in natural water than in high-purity water. The increase of ionic strength increased the mean size of particle clusters, promoting rapid sedimentation. All this indicates the importance of testing under real field conditions for attaining reliable solar to hydrogen (STH) efficiency.
Diciembre, 2023 | DOI: 10.1016/j.jece.2023.111216
Química de Superficies y Catálisis
Effect of zeolite topological structure in bifunctional catalyst on direct conversion of syngas to light olefins
Meng, FH; Gong, ZY; Yang, LL; Wang, Q; Xing, MQ; Nawaz, MA; Li, ZMicroporous and Mesoporous Materials, 362 (2023) 112792
Show abstract ▽
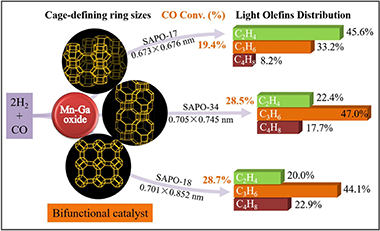
Bifunctional catalyst composed of metal oxide and zeolite (OX-ZEO) is a promising strategy for the direct conversion of syngas to light olefins (STO), where the structure of zeolite plays a vital role in determining the selectivity of product. Herein, three kinds of silicoaluminophosphate zeolites with different topological structures, i.e., the ERI(SP17), AEI(SP18) and CHA(SP34), were hydrothermally synthesized, after the combination with Mn-Ga oxide, the prepared OX-ZEO was applied for STO reaction. The variation in the crystallization time for SP17 synthesis has a great impact on the generation of impurity phase of SAPO-5, where a crystallization time of 48-96 h is found to be beneficial in synthesizing SP17 zeolite with pure phase. SP17 zeolite with a crystallization time of 96 h, possesses the micropores and columnar morphology, where the small cage-defining 8-ring size of SP17 shows the olefins selectivity of 87.0% at a low CO conversion of 19.4%, significantly deviating towards the major fraction of ethylene (45.6%) than that of butene (8.2%). In a contrast, SP18 and SP34 zeolites with the same and large cage-defining 8-ring size, are richer in propylene and butene fractions than that of ethylene in overall similar olefins selectivity of 87.0% and 87.1% at CO conversion of 28.7% and 28.5%, respectively. Interestingly, it is further interpreted that the SP17 sample generated more carbon species during the reaction due to the small 8-ring size, while those amounts of carbon species were restricted in the hierarchical pore structure and plate-like morphology in SP18 and SP34 samples.
Diciembre, 2023 | DOI: 10.1016/j.micromeso.2023.112792
- ‹ anterior
- 6 of 410
- siguiente ›














