Artículos SCI
2021
2021
Química de Superficies y Catálisis
Assessing the impact of textural properties in Ni-Fe catalysts for CO2 methanation performance
Gonzalez-Castano, M; de Miguel, JCN; Boelte, JH; Centeno, MA; Klepel, O; Arellano-Garcia, HMicroporous and Mesoporous Materials, 327 (2021) 111405
Show abstract ▽
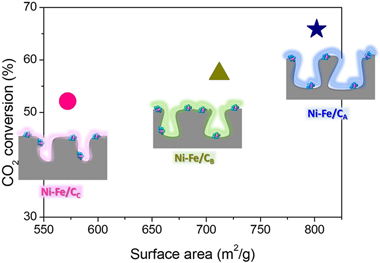
In heterogeneous catalysis, the benefits of employing adequate textural properties on the catalytic performances are usually stated. Nevertheless, the quantification of the extent of improvement is not an easy task since variations on the catalysts' specific areas and pore structures might involve modifications on a number of other surface catalytic features. This study establishes the impact of the catalyst textural properties on the CO2 methanation performance by investigating bimetallic Ni–Fe catalysts supported over carbon supports with different textural properties regarding surface area and pore structure. The comparable metal loading and dispersions attained for all systems enabled establishing forthright relationships between the catalyst textural properties and CO2 methanation rate. Once the influence of the external mass diffusions on the catalysts’ performance was experimentally discarded, the estimated Thiele modulus and internal effectiveness (φ and ηEff) values showed that the catalyst performance was majorly governed by the surface reaction rate whilst the pore size affected in no significant manner within the examined range (Dpore = 10.2 to 5.8 nm). Therefore, the rapport between the catalyst performance and surface area was quantified for the CO2 methanation reaction over Ni–Fe catalysts: increasing the surface area from 572 to 802 m2/g permit obtaining ca. 10% higher CO2 conversions.
Noviembre, 2021 | DOI: 10.1016/j.micromeso.2021.111405
Materiales de Diseño para la Energía y Medioambiente
Waterproof-breathable films from multi-branched fluorinated cellulose esters
Tedeschi, G; Guzman-Puyol, S; Ceseracciu, L; Benitez, JJ; Goldoni, L; Koschella, A; Heinze, T; Cavallo, G; Dichiarante, V; Terraneo, G; Athanassiou, A; Metrangolo, P; Heredia-Guerrero, JACarbohydrate Polymers, 271 (2021) 118031
Show abstract ▽
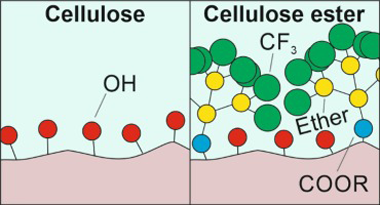
Cellulose ester films were prepared by esterification of cellulose with a multibranched fluorinated carboxylic acid, "BRFA" (BRanched Fluorinated Acid), at different anhydroglucose unit:BRFA molar ratios (i.e., 1:0, 10:1, 5:1, and 1:1). Morphological and optical analyses showed that cellulose-BRFA materials at molar ratios 10:1 and 5:1 formed flat and transparent films, while the one at 1:1 M ratio formed rough and translucent films. Degrees of substitution (DS) of 0.06, 0.09, and 0.23 were calculated by NMR for the samples at molar ratios 10:1, 5:1, and 1:1, respectively. ATR-FTIR spectroscopy confirmed the esterification. DSC thermograms showed a single glass transition, typical of amorphous polymers, at -11 degrees C. The presence of BRFA groups shifted the mechanical behavior from rigid to ductile and soft with increasing DS. Wettability was similar to standard fluoropolymers such as PTFE and PVDF. Finally, breathability and water uptake were characterized and found comparable to materials typically used in textiles.
Noviembre, 2021 | DOI: 10.1016/j.carbpol.2021.118031
Materiales Ópticos Multifuncionales
Nanophotonics for current and future white light-emitting devices
Galisteo-Lopez, JF; Lozano, GJournal of Applied Physics, 130 (2021) 200901
Show abstract ▽
Photonic nanostructures have proven useful to enhance the performance of a wide variety of materials and devices for sensing, catalysis, light harvesting, or light conversion. Herein, we discuss the role of nanophotonics in current and next-generation designs of white light-emitting diodes (LEDs). We discuss recent developments on luminescent materials designed as alternatives to rare earth-doped inorganic microcrystals, i.e., phosphors, for color conversion in LEDs, which has opened the door to the integration of resonant photonic architectures. Nanophotonics enables the devised light-matter interaction with luminescent materials in the nanoscale, which allows providing emitting devices with both enhanced performance and novel functionalities to tackle technological challenges
Noviembre, 2021 | DOI: 10.1063/5.0065825
Materiales de Diseño para la Energía y Medioambiente
Zinc Polyaleuritate Ionomer Coatings as a Sustainable, Alternative Technology for Bisphenol A-Free Metal Packaging
Morselli, D; Cataldi, P; Paul, UC; Ceseracciu, L; Benitez, JJ; Scarpellini, A; Guzman-Puyol, S; Heredia, A; Valentini, P; Pompa, PP; Marrero-López, D; Athanassiou, A; Heredia-Guerrero, AACS Sustainable Chemistry & Engineering, 9 (2021) 15484-15495
Show abstract ▽
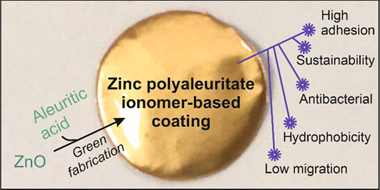
Sustainable coatings for metal food packaging were prepared from ZnO nanoparticles (obtained by the thermal decomposition of zinc acetate) and a naturally occurring polyhydroxylated fatty acid named aleuritic (or 9,10,16-trihydroxy-hexadecanoic) acid. Both components reacted, originating under specific conditions zinc polyaleuritate ionomers. The polymerization of aleuritic acid into polyaleuritate by a solvent-free, melt polycondensation reaction was investigated at different times (15, 30, 45, and 60 min), temperatures (140, 160, 180, and 200 degrees C), and proportions of zinc oxide and aleuritic acid (0:100, 5:95, 10:90, and 50:50, w/w). Kinetic rate constants calculated by infrared spectroscopy decreased with the amount of Zn due to the consumption of reactive carboxyl groups, while the activation energy of the polymerization decreased as a consequence of the catalyst effect of the metal. The adhesion and hardness of coatings were determined from scratch tests, obtaining values similar to robust polymers with high adherence. Water contact angles were typical of hydrophobic materials with values >= 94 degrees. Both mechanical properties and wettability were better than those of bisphenol A (BPA)-based resins and most likely are related to the low migration values determined using a hydrophilic food simulant. The presence of zinc provided a certain degree of antibacterial properties. The performance of the coatings against corrosion was studied by electrochemical impedance spectroscopy at different immersion times in an aqueous solution of NaCl. Considering the features of these biobased lacquers, they can be potential materials for bisphenol A-free metal packaging.
Noviembre, 2021 | DOI: 10.1021/acssuschemeng.1c04815
Nanotecnología en Superficies y Plasma
Recent Advances in Alkaline Exchange Membrane Water Electrolysis and Electrode Manufacturing
Lopez-Fernandez, E; Sacedon, CG; Gil-Rostra, J; Yubero, F; Gonzalez-Elipe, AR; De Lucas-Consuegra, AMolecules, 26 (2021) 6326
Show abstract ▽
Water electrolysis to obtain hydrogen in combination with intermittent renewable energy resources is an emerging sustainable alternative to fossil fuels. Among the available electrolyzer technologies, anion exchange membrane water electrolysis (AEMWE) has been paid much attention because of its advantageous behavior compared to other more traditional approaches such as solid oxide electrolyzer cells, and alkaline or proton exchange membrane water electrolyzers. Recently, very promising results have been obtained in the AEMWE technology. This review paper is focused on recent advances in membrane electrode assembly components, paying particular attention to the preparation methods for catalyst coated on gas diffusion layers, which has not been previously reported in the literature for this type of electrolyzers. The most successful methodologies utilized for the preparation of catalysts, including co-precipitation, electrodeposition, sol-gel, hydrothermal, chemical vapor deposition, atomic layer deposition, ion beam sputtering, and magnetron sputtering deposition techniques, have been detailed. Besides a description of these procedures, in this review, we also present a critical appraisal of the efficiency of the water electrolysis carried out with cells fitted with electrodes prepared with these procedures. Based on this analysis, a critical comparison of cell performance is carried out, and future prospects and expected developments of the AEMWE are discussed.
Noviembre, 2021 | DOI: 10.3390/molecules26216326
- ‹ anterior
- 65 of 410
- siguiente ›














