Artículos SCI
2013
2013
Materiales Nanoestructurados y Microestructura
Characterisation of Co@Fe3O4 core@shell nanoparticles using advanced electron microscopy
Knappett, BR; Abdulkin, P; Ringe, E; Jefferson, DA; Lozano-Perez, S; Rojas, TC; Fernandez, A; Wheatley, AEHNanoscale, 5 (2013) 5765-5772
Show abstract ▽
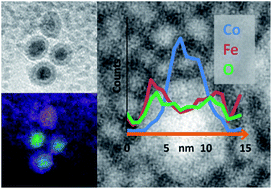
Cobalt nanoparticles were synthesised via the thermal decomposition of Co2(CO)8 and were coated in iron oxide using Fe(CO)5. While previous work focused on the subsequent thermal alloying of these nanoparticles, this study fully elucidates their composition and core@shell structure. State-of-the-art electron microscopy and statistical data processing enabled chemical mapping of individual particles through the acquisition of energy-filtered transmission electron microscopy (EFTEM) images and detailed electron energy loss spectroscopy (EELS) analysis. Multivariate statistical analysis (MSA) has been used to greatly improve the quality of elemental mapping data from core@shell nanoparticles. Results from a combination of spatially resolved microanalysis reveal the shell as Fe3O4 and show that the core is composed of oxidatively stable metallic Co. For the first time, a region of lower atom density between the particle core and shell has been observed and identified as a trapped carbon residue attributable to the organic capping agents present in the initial Co nanoparticle synthesis.
Julio, 2013 | DOI: 10.1039/C3NR33789H
Materiales de Diseño para la Energía y Medioambiente
Monolayer arrangement of fatty hydroxystearic acids on graphite: Influence of hydroxyl groups
Medina, S; Benitez, JJ; Castro, MA; Cerrillos, C; Millan, C; Alba, MDThin Solid Films, 539 (2013) 194-200
Show abstract ▽
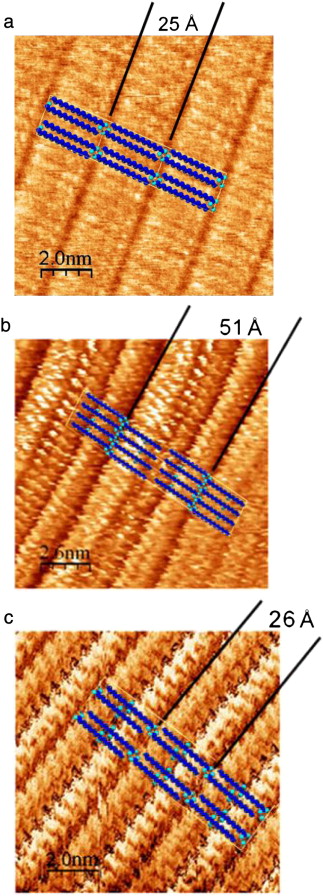
Previous studies have indicated that long-chain linear carboxylic acids form commensurate packed crystalline monolayers on graphite even at temperatures above their melting point. This study examines the effect on the monolayer formation and structure of adding one or more secondary hydroxyl, functional groups to the stearic acid skeleton (namely, 12-hydroxystearic and 9,10-dihydroxystearic acid). Moreover, a comparative study of the monolayer formation on recompressed and monocrystalline graphite has been performed through X-ray diffraction (XRD) and Scanning Tunneling Microscopy (STM), respectively. The Differential Scanning Calorimetry (DSC) and XRD data were used to confirm the formation of solid monolayers and XRD data have provided a detailed structural analysis of the monolayers in good correspondence with obtained STM images. DSC and XRD have demonstrated that, in stearic acid and 12-hydroxystearic acid adsorbed onto graphite, the monolayer melted at a higher temperature than the bulk form of the carboxylic acid. However, no difference was observed between the melting point of the monolayer and the bulk form for 9,10-dihydroxystearic acid adsorbed onto graphite. STM results indicated that all acids on the surface have a rectangular p2 monolayer structure, whose lattice parameters were uniaxially commensurate on the a-axis. This structure does not correlate with the initial structure of the pure compounds after dissolving, but it is conditioned to favor a) hydrogen bond formation between the carboxylic groups and b) formation of hydrogen bonds between secondary hydroxyl groups, if spatially permissible. Therefore, the presence of hydroxyl functional groups affects the secondary structure and behavior of stearic acid in the monolayer.
Julio, 2013 | DOI: 10.1016/j.tsf.2013.05.053
Nanotecnología en Superficies y Plasma
Liquids Analysis with Optofluidic Bragg Microcavities
Oliva-Ramirez, M; Gonzalez-Garcia, L; Parra-Barranco, J; Yubero, F; Barranco, A; Gonzalez-Elipe, ARACS Applied Materials & Interfaces, 5 (2013) 6743-650
Show abstract ▽
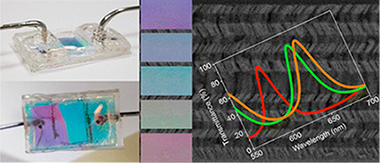
Porous Bragg microcavities formed by stacking a series of porous nanocolumnar layers with alternate low (SiO2) and high (TiO2) refractive index materials have been prepared by physical vapor deposition at glancing angles (GLAD). By strictly controlling the porosity and refractive index of the individual films, as well as the relative orientation of the nanocolumns from one layer to the next, very porous and nondispersive high optical quality microcavities have been manufactured. These photonic structures have been implemented into responsive devices to characterize liquids, mixtures of liquids, or solutions flowing through them. The large displacements observed in the optical spectral features (Bragg reflector gap and resonant peak) of the photonic structures have been quantitatively correlated by optical modeling with the refractive index of the circulating liquids. Experiments carried out with different glucose and NaCl solutions and mixtures of water plus glycerol illustrate the potentialities of these materials to serve as optofluidic devices to determine the concentration of solutions or the proportion of two phases in a liquid mixture.
Julio, 2013 | DOI: 10.1021/am401685r
Nanotecnología en Superficies y Plasma
Preparation and characterization of CrO2 films by Low Pressure Chemical Vapor Deposition from CrO3
Aguilera, C; Gonzalez, JC; Borras, A; Margineda, D; Gonzalez, JM; Gonzalez-Elipe, AR; Espinos, JPThin Solid Films, 539 (2013) 1-11
Show abstract ▽
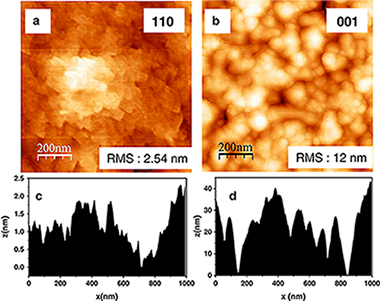
Highly oriented CrO2 thin films have been heteroepitaxially grown on TiO2 rutile (110), (100) and (001) single crystalline substrates, by Low Pressure Chemical Vapor Deposition from CrO3 as precursor and flowing oxygen as carrier gas, under a pressure of 67 Pa. The experimental conditions were fine tuned by depositing on polycrystalline Ti foils, to improve the purity of the films and the deposition rate. A maximum deposition rate of 175 nm h− 1 was obtained.
The composition and texture of films, up to 2 μm thick, have been determined by X-ray diffraction (XRD) and Micro Raman, while their microstructure has been examined by Scanning Electron Microcopy and Atomic Force Microscopy, and their magnetic behavior has been tested by superconducting quantum interference device magnetometry. These techniques reveal that the phase purity, texture, microstructure and thickness of these films are dependent on the crystalline face of the rutile substrate and the deposition temperature. Thus, microscopy techniques, XRD and Raman spectroscopy confirm that highly textured CrO2 films were always obtained on the three rutile substrate faces when deposition temperature ranges between 616 K and 636 K. But these techniques also show that CrO2 films are unpurified with inclusions or patches of Cr2O3, for the most of the substrates and especially at high deposition temperatures. Magnetic measurements conclusively demonstrate that pure CrO2 films are only obtained when TiO2 (110) is used as a substrate.
Julio, 2013 | DOI: 10.1016/j.tsf.2013.04.118
Reactividad de Sólidos
CO2 multicyclic capture of pretreated/doped CaO in the Ca-looping process. Theory and experiments
Valverde, JM; Sanchez-Jimenez, PE; Perejon, A; Perez-Maqueda, LAPhysical Chemistry Chemical Physics, 15 (2013) 11775-11793
Show abstract ▽
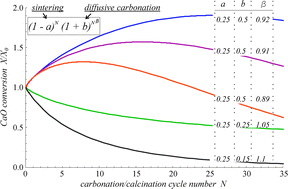
We study in this paper the conversion of CaO-based CO2 sorbents when subjected to repeated carbonation–calcination cycles with a focus on thermally pretreated/doped sorbents. Analytical equations are derived to describe the evolution of conversion with the cycle number from a unifying model based on the balance between surface area loss due to sintering in the looping-calcination stage and surface area regeneration as a consequence of solid-state diffusion during the looping-carbonation stage. Multicyclic CaO conversion is governed by the evolution of surface area loss/regeneration that strongly depends on the initial state of the pore skeleton. In the case of thermally pretreated sorbents, the initial pore skeleton is highly sintered and regeneration is relevant, whereas for nonpretreated sorbents the initial pore skeleton is soft and regeneration is negligible. Experimental results are obtained for sorbents subjected to a preheating controlled rate thermal analysis (CRTA) program. By applying this preheating program in a CO2 enriched atmosphere, CaO can be subjected to a rapid carbonation followed by a slow rate controlled decarbonation, which yields a highly sintered skeleton displaying a small conversion in the first cycle and self-reactivation in the next ones. Conversely, carbonation of the sorbent at a slow controlled rate enhances CO2 solid-state diffusion, which gives rise, after a quick decarbonation, to a highly porous skeleton. In this case, CaO conversion in the first cycle is very large but it decays abruptly in subsequent cycles. Data for CaO conversion retrieved from the literature and from further experimental measurements performed in our work are analyzed as influenced by a variety of experimental variables such as preheating temperature program, preheating exposition time, atmosphere composition, presence of additives, and carbonation–calcination conditions. Conversion data are well fitted by the proposed model equations, which are of help for a quantitative interpretation of the effect of experimental conditions on the multicyclic sorbent performance as a function of sintering/regeneration parameters inferred from the fittings and allow foreseeing the critical conditions to promote reactivation. The peculiar behavior of some pretreated sorbents, showing a maximum conversion in a small number of cycles, is explained in light of the model.
Julio, 2013 | DOI: 10.1039/C3CP50480H
- ‹ anterior
- 301 of 422
- siguiente ›














