Artículos SCI
2024
2024
Química de Superficies y Catálisis
Biomass gasification, catalytic technologies and energy integration for production of circular methanol: New horizons for industry decarbonisation
Bobadilla, Luis F; Azancot, Lola; González-Castaño, Miriam; Ruíz-López, Estela; Pastor-Pérez, Laura; Durán-Olivencia, Francisco J.; Ye, Runping; Chong, Katie; Blanco-Sánchez, Paula H; Wu, Zenthao; Reina, TR; Odriozola, JAJournal of Environmental Sciences, 140 (2024) 306-318
Show abstract ▽

The Intergovernmental Panel on Climate Change (IPCC) recognises the pivotal role of renewable energies in the future energy system and the achievement of the zero-emission target. The implementation of renewables should provide major opportunities and enable a more secure and decentralised energy supply system. Renewable fuels provide long-term solutions for the transport sector, particularly for applications where fuels with high energy density are required. In addition, it helps reducing the carbon footprint of these sectors in the long-term. Information on biomass characteristics feedstock is essential for scaling-up gasification from the laboratory to industrial-scale. This review deals with the transformation biogenic residues into a valuable bioenergy carrier like biomethanol as the liquid sunshine based on the combination of modified mature technologies such as gasification with other innovative solutions such as membranes and microchannel reactors. Tar abatement is a critical process in product gas upgrading since tars compromise downstream processes and equipment, for this, membrane technology for upgrading syngas quality is discussed in this paper. Microchannel reactor technology with the design of state-of-the-art multifunctional catalysts provides a path to develop decentralised biomethanol synthesis from biogenic residues. Finally, the development of a process chain for the production of (i) methanol as an intermediate energy carrier, (ii) electricity and (iii) heat for decentralised applications based on biomass feedstock flexible gasification, gas upgrading and methanol synthesis is analysed.
Junio, 2024 | DOI: 10.1016/j.jes.2023.09.020
Química de Superficies y Catálisis
Nickel-based cerium zirconate inorganic complex structures for CO2 valorisation via dry reforming of methane
Martín-Espejo, Juan Luis; Merkouri, Loukia-Pantzechroula; Gándara-Loe, Jesús; Odriozola, José AntonioJournal of Environmental Sciences
Show abstract ▽
The increasing anthropogenic emissions of greenhouse gases (GHG) is encouraging extensive research in CO2 utilisation. Dry reforming of methane (DRM) depicts a viable strategy to convert both CO2 and CH4 into syngas, a worthwhile chemical intermediate. Among the different active phases for DRM, the use of nickel as catalyst is economically favourable, but typically deactivates due to sintering and carbon deposition. The stabilisation of Ni at different loadings in cerium zirconate inorganic complex structures is investigated in this work as strategy to develop robust Ni-based DRM catalysts. XRD and TPR-H2 analyses confirmed the existence of different phases according to the Ni loading in these materials. Besides, superficial Ni is observed as well as the existence of a CeNiO3 perovskite structure. The catalytic activity was tested, proving that 10 wt.% Ni loading is the optimum which maximises conversion. This catalyst was also tested in long-term stability experiments at 600 and 800°C in order to study the potential deactivation issues at two different temperatures. At 600°C, carbon formation is the main cause of catalytic deactivation, whereas a robust stability is shown at 800°C, observing no sintering of the active phase evidencing the success of this strategy rendering a new family of economically appealing CO2 and biogas mixtures upgrading catalysts.
Junio, 2024 | DOI: 10.1016/j.jes.2023.01.022
Química de Superficies y Catálisis
Hydrochar and synthetic natural gas co-production for a full circular economy implementation via hydrothermal carbonization and methanation: An economic approach
Judith González-Arias, Guillermo Torres-Sempere, Miriam González-Castaño, Francisco M. Baena-Moreno, Tomás R. ReinaJournal of Environmental Sciences
Show abstract ▽
Herein we study the economic performance of hydrochar and synthetic natural gas co-production from olive tree pruning. The process entails a combination of hydrothermal carbonization and methanation. In a previous work, we evidenced that standalone hydrochar production via HTC results unprofitable. Hence, we propose a step forward on the process design by implementing a methanation, adding value to the gas effluent in an attempt to boost the overall process techno-economic aspects. Three different plant capacities were analyzed (312.5, 625 and 1250 kg/hr). The baseline scenarios showed that, under the current circumstances, our circular economy strategy in unprofitable. An analysis of the revenues shows that hydrochar selling price have a high impact on NPV and subsidies for renewable coal production could help to boost the profitability of the process. On the contrary, the analysis for natural gas prices reveals that prices 8 times higher than the current ones in Spain must be achieved to reach profitability. This seems unlikely even under the presence of a strong subsidy scheme. The costs analysis suggests that a remarkable electricity cost reduction or electricity consumption of the HTC stage could be a potential strategy to reach profitability scenarios. Furthermore, significant reduction of green hydrogen production costs is deemed instrumental to improve the economic performance of the process. These results show the formidable techno-economic challenge that our society faces in the path towards circular economy societies.
Junio, 2024 | DOI: 10.1016/j.jes.2023.04.019
Reactividad de Sólidos
Ca-based materials derived from calcined cigarette butts for CO2 capture and thermochemical energy storage
Amghar, N; Moreno, V; Sánchez-Jiménez, PE; Perejón, A; Pérez-Maqueda, LAJournal of Environmental Sciences, 140 (2024) 230-241
Show abstract ▽
Cigarette butts (CBs) are one of the most common types of litter in the world. Due to the toxic substances they contain, the waste generated poses a harmful risk to the environment, and therefore there is an urgent need for alternative solutions to landfill storage. Thus, this work presents a possible revalorization of this waste material, which implies interesting environmental benefits. CBs were used as sacrificial templates for the preparation of CaO-based materials by impregnation with calcium and magnesium nitrates followed by flaming combustion. These materials presented enhanced porosity for their use in the Calcium Looping process applied either to thermochemical energy storage or CO2 capture applications. The influence of the concentration of Ca and Mg in the impregnating solutions on the multicycle reactivity of the samples was studied. An improved multicycle performance was obtained in terms of conversion for both applications.
Junio, 2024 | DOI: 10.1016/j.jes.2023.07.028
Fotocatálisis Heterogénea: Aplicaciones
Photocatalytic activity enhancement by noble metal deposition on faceted F-TiO2 synthesised by microwave assisted method. A study of selective oxidation of gas-phase ethanol in a FBPR reactor
Hernández-Laverde, M; Murcia, JJ; Morante, N; Sannino, D; Vaiano, V; Navío, JA; Hidalgo, MCCatalysis Today, 433 (2024) 114645
Show abstract ▽
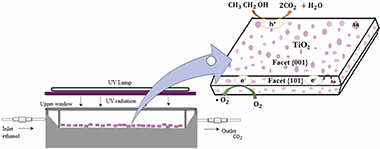
In the present work, fluorinated titanium dioxide (TiO2-F) with high exposition of facet {001} was prepared by following a facile and high yield hydrothermal method assisted by microwave. This faceted TiO2 was then modified by Au or Ag deposition at two different metal loadings (0.125 and 0.25 wt%). A wide physicochemical characterisation of the materials was performed. X-ray difractograms showed high {001} facet exposition in all materials. By X-ray fluorescence it was found that the different samples contained about 5% of fluor. All samples presented high surface area and high uniformity and homogeneity of particles, which highlights the good properties that can be achieved by the microwave synthesis method compared to conventional hydrothermal methods. Oxidation state of the noble metals was studied by XPS. On the other side, TiO2-F and the metallised titania powders were immobilised on polystyrene pellets (PS) for evaluating their gas photocatalytic activity in volatile organic compounds (VOCs) decontamination by following the reaction of photoxidation of ethanol in gas phase. It was found that activity was considerably improved by the addition of noble metals, obtaining high conversion and selectivity to CO2. It is remarkable that the selectivity to CO is almost zero. The highest efficiency was found for the faceted TiO2-F sample with the lowest Au loading (0.125 wt%) immobilised on PS where 91% ethanol conversion and 100% CO2 selectivity were achieved. Different reaction variables were also studied.
Mayo, 2024 | DOI: 10.1016/j.cattod.2024.114645
- 1 of 410
- siguiente ›














