Scientific Papers in SCI
2021
2021
Materiales de Diseño para la Energía y Medioambiente
Structural Evolution in Iron-Catalyzed Graphitization of Hard Carbons
Gomez-Martin, A; Schnepp, Z; Ramirez-Rico, JChemistry of Materials, 33 (2021) 3087-3097
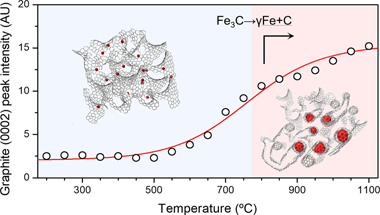
Despite the recent interest in catalytic graphitization to obtain graphite-like materials from hard-carbon sources, many aspects of its mechanism are still poorly unknown. We performed a series of in situ experiments to study phase transformations during graphitization of a hard-carbon precursor using an iron catalyst at temperatures up to 1100 degrees C and ex situ total scattering experiments up to 2000 degrees C to study the structural evolution of the resulting graphitized carbon. Our results show that upon heating and cooling, iron undergoes a series of reductions to form hematite, magnetite, and wustite before forming a carbide that later decomposes into metallic iron and additional graphite and that the graphitization fraction increases with increasing peak temperature. Structural development with temperature results in decreasing sheet curvature and increased stacking, along with a decrease in turbostratic disorder up to 1600 degrees C. Higher graphitization temperatures result in larger graphitic domains without further ordering of the graphene sheets. Our results have implications for the synthesis of novel biomass-derived carbon materials with enhanced crystallinity.
May, 2021 | DOI: 10.1021/acs.chemmater.0c04385
Materiales Avanzados
Synthesis of clay geopolymers using olive pomace fly ash as an alternative activator. Influence of the additional commercial alkaline activator used
Gomez-Casero, MA; Moral-Moral, FJ; Perez-Villarejo, L; Sanchez-Soto, PJ; Eliche-Quesada, DJournal of Materials Research and Technology-JMR&T 12 (2021) 1762-1776
In this research, the use of olive pomace fly ash (OPFA) as an alkaline source for the activation of calcined clays (CC) from Bailen (Jaen, Spain) was studied. The optimal composition was obtained for 70 wt % CC and 30 wt % OPFA. The physical, mechanical and thermal properties of control geopolymers that use water as a liquid medium have been studied and compared with geopolymers that use additional activating solutions as sodium or potassium hydroxide solutions (8 M), or a mixture of alkaline hydroxide and alkaline silicate solution (NaOH-Na2SiO3 or KOH-K2SiO3). The results showed that OPFA can be used as an alkaline activator, showing mechanical properties slightly lower than those obtained when additional alkaline hydroxide activating solutions were used. The best compressive strength was obtained for geopolymers that use alkaline silicates as an activating solution. However, the best thermal insulation properties were obtained for control geopolymers. The microstructural characteristics of the geopolymers were evaluated by means of X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy and Scanning Electron Microscopy (SEM-EDS) that corroborate the formation of geopolymeric gel in all the specimens, being the amount of gel formed greater in samples using commercial potassium activating solutions. These results demonstrate the feasibility of using this type of waste, OPFA, as activating reagents in the manufacture of geopolymers or alkaline activated materials. The manufactured geopolymers can be used as compressed earth blocks for walls and partitions, since the specimens pursue mechanical properties that comply with current regulations, presenting better thermal insulation properties.
May, 2021 | DOI: 10.1016/j.jmrt.2021.03.102
Química de Superficies y Catálisis
Understanding the opportunities of metal-organic frameworks (MOFs) for CO2 capture and gas-phase CO2 conversion processes: a comprehensive overview
Gandara-Loe, J; Pastor-Perez, L; Bobadilla, LF; Odriozola, JA; Reina, TRReaction Chemistry & Engineering, 6 (2021) 787-814

The rapid increase in the concentration of atmospheric carbon dioxide is one of the most pressing problems facing our planet. This challenge has motivated the development of different strategies not only in the reduction of CO2 concentrations via green energy alternatives but also in the capture and conversion of CO2 into value-added products. Metal-organic frameworks (MOFs) are a relatively new class of porous materials with unique structural characteristics such as high surface areas, chemical tunability and stability, and have been extensively studied as promising materials to address this challenge. This comprehensive review identifies the specific structural and chemical properties of MOFs that result in advanced CO2 capture capacities and fairly encouraging catalytic CO2 conversion behaviour. More importantly, we describe an interconnection among the unique properties of MOFs and the engineering aspects of these intriguing materials towards CO2 capture and conversion processes.
May, 2021 | DOI: 10.1039/d1re00034a
Mössbauer study of iron gall inks on historical documents
Lerf, A; Wagner, FE; Dreher, M; Espejo, T; Perez-Rodriguez, JLHeritage Science, 9 (2021) 49
Iron gall ink was used in the Western world as a permanent writing material already in late Roman times and throughout the Middle Ages, until it became obsolete in the twentieth century. There is much interest in experimental methods to determine the state of the ink and its degradation products on historical documents. Mossbauer spectroscopy with Fe-57 is such a method, and it has the particular advantage to be sensitive to the chemical bonding of iron, but this method has only rarely been applied to historical documents. In this paper we present Mossbauer data for two damaged documents from a Library in Granada and a handwritten German book from the eighteenth century. In addition to the inked parts of the manuscripts, ink-free parts were studied to determine the amount and chemical state of the iron in the papers. These new results are discussed in the context of previously published Mossbauer data. In one of the investigated documents Fe(II)-oxalate, FeC2O4 center dot 2H(2)O, was observed. The assignment of the various Fe3+ sites in the different documents is rather difficult and often there is a superposition of various species. Known forms of iron gallate are definitely not present on the inked papers. The observed ferric species can be remains of Fe3+ polyphenol complexes of the ink, complexes of Fe3+ with degradation products of the cellulose of the paper or gum arabic, or very small iron oxide or hydroxide nanoparticles.
May, 2021 | DOI: 10.1186/s40494-021-00522-3
Tribología y Protección de Superficies - Materiales Ópticos Multifuncionales
High-temperature solar-selective coatings based on Cr(Al)N. Part 1: Microstructure and optical properties of CrNy and Cr1-xAlxNy films prepared by DC/HiPIMS
Rojas, TC; Caro, A; Lozano, G.; Sanchez-Lopez, JCSolar Energy Materials and Solar Cells, 223 (2021) 110951
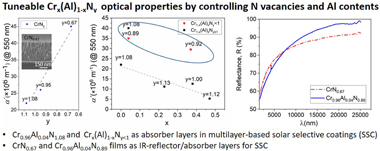
In order to explore the potentialities of Cr1-x(Al)xNy materials in multilayer-based solar selective coatings (SSC) for high temperature applications (T > 500 °C), the optical behavior of Cr1-x(Al)xNy films is studied in this work. Two sets of layers (CrNy and Cr1-xAlxNy) were prepared by direct current (DC) and high-power impulse magnetron sputtering (HiPIMS) technology. The deposition parameters: N2 flux, HiPIMS frequency and aluminum sputtering power, were modified to get a wide variety of stoichiometries. The composition, morphology, phases and electronic structure of the films were characterized in depth. The optical behavior was determined by UV–Vis–NIR and FTIR spectroscopies, and the optical constants were obtained from the measured transmittance and reflectance spectra based on appropriate dielectric function models. Our results indicate that small changes in the layer composition influence the optical constants. In particular, a metallic-like behavior was obtained for CrNy layers with N vacancies (CrN0.95 and CrN0.67 films) while a semiconductor-like behavior was observed for CrN1.08. Thus, the CrNy films can be used as effective absorber layer in multilayer-based SSC, and namely, the CrN0.67 film as an IR reflector/absorber layer too. Likewise, the optical properties of Cr1-xAlxNy layers can also be tuned from metallic to semiconductor-like behavior depending on the chemical composition. Indeed, the absorption coefficients of Cr1-xAlxNy films with optimized Al content and N-vacancies are comparable to those reported for state-of-the-art materials such as TiAlN or TiAlCrN. In addition, a Cr0.96Al0.04N0.89 film was found to be a suitable IR reflector/absorber layer.
May, 2021 | DOI: 10.1016/j.solmat.2020.110951
Química de Superficies y Catálisis
Cu supported Fe-SiO2 nanocomposites for reverse water gas shift reaction
Gonzalez-Castano, M; de Miguel, JCN; Sinha, F; Wabo, SG; Klepel, O; Arellano-Garcia, HJournal of CO2 Utilization, 46 (2021) 101493
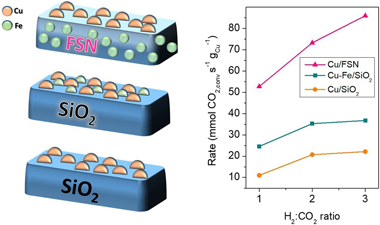
This work analyses the catalytic activity displayed by Cu/SiO2, Cu-Fe/SiO2 and Cu/FSN (Fe-SiO2 nanocomposite) catalysts for the Reverse Water Gas Shift reaction. Compared to Cu/SiO2 catalyst, the presence of Fe resulted on higher CO?s selectivity and boosted resistances against the constitution of the deactivation carbonaceous species. Regarding the catalytic performance however, the extent of improvement attained through incorporation Fe species strongly relied on the catalysts' configuration. At 30 L/gh and H-2:CO2 ratios = 3, the performance of the catalysts? series increased according to the sequence: Cu/SiO2 < Cu-Fe/SiO2 << Cu/FSN. The remarkable catalytic enhancements provided by Fe-SiO2 nanocomposites under different RWGS reaction atmospheres were associated to enhanced catalyst surface basicity's and stronger Cu-support interactions. The catalytic promotion achieved by Fe-SiO2 nanocomposites argue an optimistic prospective for nanocomposite catalysts within future CO2-valorising technologies.
April, 2021 | DOI: 10.1016/j.jcou.2021.101493
Materiales para Bioingeniería y Regeneración Tisular
Nanofibrous Matrix of Defined Composition Sustains Human Induced Pluripotent Stem Cell Culture
Borrego-Gonzalez, S; de la Cerda, B; Diaz-Corrales, FJ; Diaz-Cuenca, AACS Applied Bio Materials, 4 (2021) 3035-3040

Human induced pluripotent stem cells (hiPSCs) represent the most promising biological material for regenerative medicine applications. In this work, a 3D solid nanofibrous matrix of defined composition (Colamigel-S) consisting of 97 wt % gelatin, 2.6 wt % atelocollagen, and 0.4 wt % laminin has been reproducibly processed and characterized and exhibits a homogeneous nanofibrillar network of high surface area, interconnected microcavities, and typical D-periodic collagen fibril nanostructural features. The purpose of the study was to test the performance of Colamigel-S as substrate for in vitro hiPSCs culture, finding that these cells efficiently attach and grow keeping their characteristic stem morphology and undifferentiated state.
April, 2021 | DOI: 10.1021/acsabm.0c00425
Química de Superficies y Catálisis
Fructose dehydration reaction over functionalized nanographitic catalysts in MIBK/H2O biphasic system
Martin, GD; Bounoukta, CE; Ammari, F; Dominguez, MI; Monzon, A; Ivanova, S; Centeno, MACatalysis Today, 366 (2021) 68-76
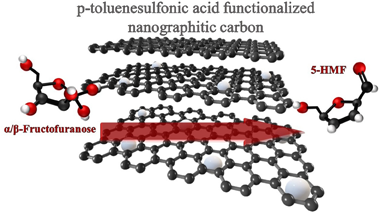
A series of functionalized nanographitic carbons is prepared, characterized and tested in fructose dehydration reaction to 5-hydroxymethylfurfural. The functionalization treatment was selected to introduce various Bro?nsted acid sites and to modify the textural and catalytic properties of the initial carbon material. Within the series, the sulfonated carbons present the most interesting catalytic behavior resulting in important selectivity to the desired product once the reaction variables were properly adjusted.
April, 2021 | DOI: 10.1016/j.cattod.2020.03.016
Química de Superficies y Catálisis
Biogas Conversion to Syngas Using Advanced Ni-Promoted Pyrochlore Catalysts: Effect of the CH4/CO2 Ratio
le Sache, E; Moreno, AA; Reina, TRFrontiers in Chemistry, 9 (2021) 672419
Biogas is defined as the mixture of CH4 and CO2 produced by the anaerobic digestion of biomass. This particular mixture can be transformed in high valuable intermediates such as syngas through a process known as dry reforming (DRM). The reaction involved is highly endothermic, and catalysts capable to endure carbon deposition and metal particle sintering are required. Ni-pyrochlore catalysts have shown outstanding results in the DRM. However, most reported data deals with CH4/CO2 stoichiometric ratios resulting is a very narrow picture of the overall biogas upgrading via DRM. Therefore, this study explores the performance of an optimized Ni-doped pyrochlore, and Ni-impregnated pyrochlore catalysts in the dry reforming of methane, under different CH4/CO2 ratios, in order to simulate various representatives waste biomass feedstocks. Long-term stability tests showed that the ratio CH4/CO2 in the feed gas stream has an important influence in the catalysts' deactivation. Ni doped pyrochlore catalyst, presents less deactivation than the Ni-impregnated pyrochlore. However, biogas mixtures with a CH4 content higher than 60%, lead to a stronger deactivation in both Ni-catalysts. These results were in agreement with the thermogravimetric analysis (TGA) of the post reacted samples that showed a very limited carbon formation when using biogas mixtures with CH4 content <60%, but CH4/CO2 ratios higher than 1.25 lead to an evident carbon deposition. TGA analysis of the post reacted Ni impregnated pyrochlore, showed the highest amount of carbon deposited, even with lower stoichiometric CH4/CO2 ratios. The later result indicates that stabilization of Ni in the pyrochlore structure is vital, in order to enhance the coke resistance of this type of catalysts.
April, 2021 | DOI: 10.3389/fchem.2021.672419
Reactividad de Sólidos
Enhancing the electrical conductivity of in-situ reduced graphene oxide-zirconia composites through the control of the processing routine
Lopez-Pernia, C; Morales-Rodriguez, A; Gallardo-Lopez, A; Poyato, RCeramics International, 47 (2021) 9382-9391
Graphene oxide (GO) was mixed with 3 mol% yttria tetragonal zirconia polycrystal (3YTZP) using two powder processing routines: a colloidal method in an aqueous solution and a combination of ultrasonication with highenergy planetary ball milling in wet conditions. Highly densified 3YTZP composites with reduced GO (rGO) were consolidated by Spark Plasma Sintering. The in-situ reduction of GO was successfully achieved during the high temperature sintering process and a detailed study of the restoration of the graphene structure in the sintered composites has been made by Raman spectroscopy. Although no differences between the composites prepared by the two processing methods were found in the distribution of the rGO throughout the 3YTZP matrix for high rGO contents (i.e. the composites with 5 and 10 vol% rGO), a better distribution of the graphene phase was found in the composites with 1 and 2.5 vol% rGO prepared by planetary ball milling. This result, together with a better reduction of the GO in these composites, led to the obtaining of rGO/3YTZP composites with a better behavior in terms of electrical conductivity: an electrical percolation threshold below 2.5 vol% rGO and a high electrical conductivity value (-610 S/m for 10 vol% rGO).
April, 2021 | DOI: 10.1016/j.ceramint.2020.12.069
- ‹ previous
- 48 of 214
- next ›














