Artículos SCI
2018
2018
Tribología y Protección de Superficies
Tribological properties of TiC/a-C:H nanocomposite coatings prepared via HiPIMS
Sanchez-Lopez, JC; Dominguez-Meister, S; Rojas, TC; Colasuonno, M; Bazzan, M; Patelli, AApplied Surface Science, 440 (2018) 458-466
Show abstract ▽
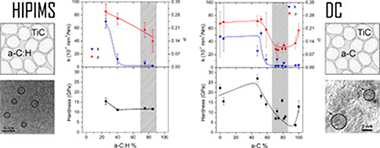
High power impulse magnetron sputtering (HiPIMS) technology has been employed to prepare TiC/a-C:H nanocomposite coatings from a titanium target in acetylene (C2H2) reactive atmospheres. Gas fluxes were varied from 1.3 to 4.4 sccm to obtain C/Ti ratios from 2 to 15 as measured by electron probe microanalysis (EPMA). X-ray diffraction and transmission electron microscopy demonstrate the presence of TiC nanocrystals embedded in an amorphous carbon-based matrix. The hardness properties decrease from 17 to 10 GPa as the carbon content increases. The tribological properties were measured using a pinon-disk tribometer in ambient air (RH = 30-40%) at 10 cm/s with 5 N of applied load against 6-mm 100Cr6 balls. The friction coefficient and the film wear rates are gradually improved from 0.3 and 7 x 10(-6) mm(3)/N m to 0.15 and 2 x 10(-7) mm(3)/N m, respectively, by increasing the C2H2 flux. To understand the tribological processes appearing at the interface and to elucidate the wear mechanism, microstructural and chemical investigations of the coatings were performed before and after the friction test. EPMA, X-ray photoelectron and electron energy-loss spectroscopies were employed to obtain an estimation of the fraction of the a-C:H phase, which can be correlated with the tribological behavior. Examination of the friction counterfaces (ball and track) by Raman microanalysis reveals an increased ordering of the amorphous carbon phase concomitant with friction reduction. The tribological results were compared with similar TiC/a-C(:H) composites prepared by the conventional direct current process.
Mayo, 2018 | DOI: 10.1016/j.apsusc.2018.01.135
Reactividad de Sólidos
Obituary Note: Prof. Jose Manuel Criado
Perez-Maqueda, L; Koga, N; Malek, JThermochimica Acta, 663 (2018) A1
Show abstract ▽

The late Prof. Jose Manuel Criado (1944.6.13–2018.2.27)
It is with the profoundest regret that we must report the passing of Prof. José Manuel Criado on February 27, 2018 at the age of 73. We express our most sincere condolences to his family, colleagues and friends.
Prof. Criado was born in Sevilla, Spain, on June 13th, 1944. He studied chemistry at the University of Seville and recieved his PhD from the same university under the supervision of Prof. Francisco González García and Prof. José María Trillo. He held a position as assistant professor at the Department of Inorganic Chemistry of the University of Seville from 1968 until 1972. Then, he joined the Consejo Superior de Investigaciones Científicas (CSIC) or National Research Council of Spain. In this institution he was junior researcher, senior researcher and, from 1986, full professor. Moreover, he has been visiting professor in a number of international institutions such as Stanford University (USA), CNRS Thermodynamics and Microcalorimetry Center in Marseille (France), University of Salford (UK), Macaulay Research Institute (UK), Institute of Inorganic Chemistry (Czech Republic), University of Chile (Chile). He had long-lasting collaborations with scientists from all over the world and visited labs in many countries. For many years, he used to spend some weeks abroad in the frame of collaboration projects with a number of international research groups, of special importance where his projects with the Czech Republic or Chile that lasted for over 20 years. He also served as an editorial board member of Thermochimica Acta for a long time and contributed largely to the further development of our academic field.
First research works of Prof. Criado were done within the field of heterogeneous catalysis, but very soon, he got interested in reactivity of solids and thermal analysis. Thus, most of his scientific career has been devoted to the study of kinetics of solid-state processes and mechanochemisty. He published about 240 papers in international journals. In the field of kinetics of solid-state processes, he made significant contributions, such as showing the limitations of using single linear heating rate experiments for extracting kinetic parameters, the proposal of master curves for discerning the kinetic model followed by the process or the combined analysis of experimental data obtained under different heating schedules. Moreover, after learning about the sample controlled thermal analysis (SCTA) method directly from Prof. Rouquerol in Marseille (France) and Profs. Paulik brothers in Budapest (Hungary), he constructed several of these instruments and extended the use of SCTA to the kinetic analysis of heterogeneous reactions, highlighting its advantages over conventional heating. Additionally, he used the kinetic control of solid-state processes by SCTA for the preparation of a number of functional and structural materials with controlled microstructures and properties. In the field of mechanochemistry, he made substantial contributions to the preparation of materials by gas–solid reaction using high energy planetary ball mills specially modified by him to work under controlled gas atmosphere.
Probably, the main contribution of Prof. Criado as a scientist has been as teacher and mentor for many of us. Despite of spending most of his scientific career in a research center rather than in a university, his laboratories were always full of students, postdocs and visitors from all over the world. He devoted a great effort to motivate and stimulate young people to pursue a career in science. His enthusiasm for science was sincere, as he loved science and research. Thus, he worked until the very last days and, even, when he could not go to the institute because he did not feel well, he worked in a small lab at home. He was very generous and always shared his knowledge with others. Thus, he expended long hours teaching about kinetics, making the complex equations easy to understand. Only those with a deep knowledge have this ability! It is not rare that many of his former students, postdocs and coworkers have permanent positions as professors and scientist in a number of international institutions. Another significant feature of Prof. Criado was his hospitality. He and his wife Maria Jesús Dianez, who joined his research group few years ago, has always his home doors open to any coworker or visitor.
We will all miss him not only as a scientist with a deep knowledge but as a friend we loved so much.
Mayo, 2018 | DOI: 10.1016/j.tca.2018.05.004
Química de Superficies y Catálisis
Multicomponent Ni-CeO2 nanocatalysts for syngas production from CO2/CH4 mixtures
le Sache, E.; Santos, J. L.; Smith, T. J.; Centeno, M. A.; Arellano-Garcia, H.; Odriozola, J. A.; Reina, T. R.Journal of CO2 utilization, 25 (2018) 68-78
Show abstract ▽

The dry reforming of methane with CO2 is a common route to transform CO2/CH4 mixtures into added value syngas. Ni based catalysts are highly active for this goal but suffer from deactivation, as such promoters need to be introduced to counteract this, and improve performance. In this study, mono- and bi-metallic formulations based on 10 wt.% Ni/CeO2-Al2O3 are explored and compared to a reference 10 wt.% Ni/gamma-Al2O3. The effect of Sn and Pt as promoters of Ni/CeO2-Al2O3 was also investigated. The formulation promoted with Sn looked especially promising, showing CO2 conversions stabilising at 65% after highs of 95%. Its increased performance is attributed to the additional dispersion Sn promotion causes. Changes in the reaction conditions (space velocity and temperature) cement this idea, with the Ni-Sn/CeAl material performing superiorly to the mono-metallic material, showing less deactivation. However, in the long run it is noted that the mono- metallic Ni/CeAl performs better. As such the application is key when deciding which catalyst to employ in the dry reforming process.
Mayo, 2018 | DOI: 10.1016/j.jcou.2018.03.012
Nanotecnología en Superficies y Plasma
In Vitro Comparative Study of Oxygen Plasma Treated Poly(Lactic-Co-Glycolic) (PLGA) Membranes and Supported Nanostructured Oxides for Guided Bone Regeneration Processes
Torres-Lagares, D; Castellanos-Cosano, L; Serrera-Figallo, MA; Lopez-Santos, C; Barranco, A; Rodriguez-Gonzalez-Elipe, A; Gutierrez-Perez, JLMaterials, 11 (2018) art. 752
Show abstract ▽
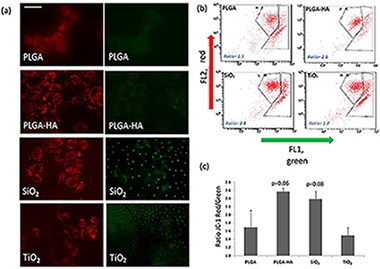
(1) Background: The use of physical barriers to prevent the invasion of gingival and connective tissue cells into bone cavities during the healing process is called guided bone regeneration. The objective of this in-vitro study was to compare the growth of human osteoblasts on Poly(Lactic-co-Glycolic) (PLGA) membranes modified with oxygen plasma and Hydroxyapatite (HA), silicon dioxide (SiO2), and titanium dioxide (TiO2) composite nanoparticles, respectively. (2) Methods: All the membranes received a common treatment with oxygen plasma and were subsequently treated with HA nanostructured coatings (n = 10), SiO2 (n = 10) and TiO2 (n = 10), respectively and a PLGA control membrane (n = 10). The assays were performed using the human osteoblast line MG-63 acquired from the Center for Scientific Instrumentation (CIC) from the University of Granada. The cell adhesion and the viability of the osteoblasts were analyzed by means of light-field microphotographs of each condition with the inverted microscope Axio Observer A1 (Carl Zeiss). For the determination of the mitochondrial energy balance, the MitoProbe (TM) JC-1 Assay Kit was employed. For the determination of cell growth and the morphology of adherent osteoblasts, two techniques were employed: staining with phalloidin-TRITC and staining with DAPI. (3) Results: The modified membranes that show osteoblasts with a morphology more similar to the control osteoblasts follow the order: PLGA/PO2/HA > PLGA/PO2/SiO2 > PLGA/PO2/TiO2 > PLGA (p < 0.05). When analysing the cell viability, a higher percentage of viable cells bound to the membranes was observed as follows: PLGA/PO2/SiO2 > PLGA/PO2/HA > PLGA/PO2/TiO2 > PLGA (p < 0.05), with a better energy balance of the cells adhered to the membranes PLGA/PO2/HA and PLGA/PO2/SiO2. (4) Conclusion: The membrane in which osteoblasts show characteristics more similar to the control osteoblasts is the PLGA/PO2/HA, followed by the PLGA/PO2/SiO2.
Mayo, 2018 | DOI: 10.3390/ma11050752
Fotocatálisis Heterogénea: Aplicaciones
Silver-modified ZnO highly UV-photoactive
Jaramillo-Páez, C.; Navío, J.C.; Hidalgo, M.C.Journal of Photochemistry and Photobiology A: Chemistry, 356 (2018) 112-122
Show abstract ▽
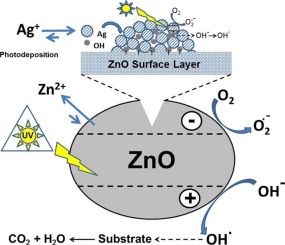
ZnO nanoparticles were successfully synthesized by a controlled precipitation procedure by mixing aqueous solutions of Zn(II) acetate and dissolved Na2CO3 at pH ca. 7.0 without template addition and ulterior calcination at 400 °C for 2 h. The Ag-ZnO catalysts (ranging from 0.5 to 10 Ag wt.-%) were obtained by photochemical deposition method at the surface of the prepared ZnO sample, using AgNO3 as precursor. The as-prepared catalysts (with and without silver) were characterized by XRD, BET, FE-SEM, TEM, and XPS and diffuse reflectance spectroscopy (DRS). The effect of Ag-phodeposition on the photocatalytic properties of ZnO nanoparticles was investigated. Three different probe molecules were used to evaluate the photocatalytic properties under UV-illumination and visible illumination: Methyl Orange and Rhodamine B were chosen as hazardous dyes and Phenol as a transparent substrate. For each of the chosen substrates, it was observed that the UV-photocatalytic properties of ZnO improved with the amount of Ag deposited, up to an optimum percentage around 1–5 wt.-% Ag, being even better than the commercial Evonik-TiO2(P25) in the same conditions. Above this amount, the UV-photocatalytic properties of the Ag-ZnO samples remain unchanged, indicating a maximum for Ag-deposition. While ZnO and Ag-ZnO catalysts can photodegrade Rhodamine B, Methyl Orange and Phenol totally within 60 min under UV-illumination, the process is slightly faster for the case of Ag–ZnO nanoparticles. Under Vis-illumination, the silver-metalized samples did not present photocatalytic activity in the degradation of Methyl Orange. However, a very low photoactivity was present for phenol degradation (10% conversion) and a moderate conversion of ca. 70% for Rhodamine B degradation, after 120 min of Visible-illumination. High conversion values and a total organic carbon (TOC) removal of 86–97% were obtained over the Ag-ZnO photocatalysts after 120 min of UV-illumination, suggesting that these Ag-modified ZnO nanoparticles may have good applications in wastewater treatment, due to its reuse properties.
Abril, 2018 | DOI: 10.1016/j.jphotochem.2017.12.044
- ‹ anterior
- 168 of 420
- siguiente ›














