Artículos SCI
2014
2014
Nanotecnología en Superficies y Plasma
Oxygen Optical Sensing in Gas and Liquids with Nanostructured ZnO Thin Films Based on Exciton Emission Detection
Sanchez-Valencia, JR; Alcaire, M; Romero-Gomez, P; Macias-Montero, M; Aparicio, FJ; Borras, A; Gonzalez-Elipe, AR; Barranco, AJournal of Physical Chemistry C, 118 (2014) 9852-9859
Show abstract ▽
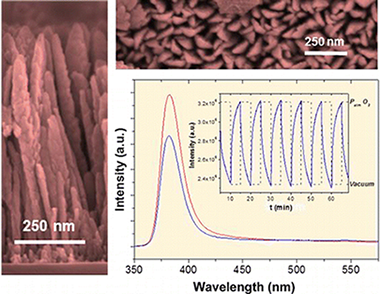
Transparent nanocolumnar porous ZnO thin films have been prepared by plasma-enhanced chemical vapor deposition. By controlling the H-2/O-2 ratio in the plasma gas, the deposition conditions were optimized to obtain an intense exciton emission at around 381 nm and virtually no luminescence in the visible region associated with electronic states in the gap. The intensity of the exciton band varied significantly and reversibly with the partial pressure of oxygen in the environment. This behavior and its variations with temperature and water vapor sustain the use of these thin films as photonic sensors of oxygen. Further experiments in liquid water show that fluorescence intensity also varies with the amount of dissolved oxygen even for concentrations lower than 0.02 mg/L where commercial oxygen galvanic sensors show limited sensitivity. These results and the use of ZnO as photonic sensor of oxygen are discussed by assuming a classical mechanism involving the photoactivated adsorption of oxygen when this oxide is irradiated with UV light during its fluorescence interrogation.
Mayo, 2014 | DOI: 10.1021/jp5026027
Química de Superficies y Catálisis
Pt vs. Au in water-gas shift reaction
Castano, MG; Reina, TR; Ivanova, S; Centeno, MA; Odriozola, JAJournal of Catalysis, 314 (2014) 1-9
Show abstract ▽
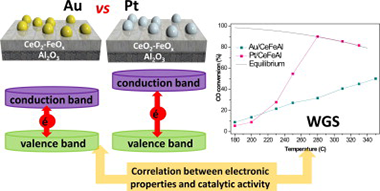
This work presents a comparison of the gold- and platinum-based catalysts behavior in the water–gas shift (WGS) reaction. The influence of the support, e.g., its composition and electronic properties, studied in detail by means of UV–Vis spectroscopy, of the metal nature and dispersion and of the stream composition has been evaluated. The catalytic performance of the samples is directly correlated with the electronic properties modification as a function of metal and/or support. Both metals present high activity in the selected reaction although in a different operation temperature window.
Mayo, 2014 | DOI: 10.1016/j.jcat.2014.03.014
Materiales de Diseño para la Energía y Medioambiente
Thermal conductivity at the amorphous-nanocrystalline phase transition in beech wood biocarbon
Parfen'eva, LS; Orlova, TS; Smirnov, BI; Smirnov, IA; Misiorek, H; Jezowski, A; Ramirez-Rico, JPhysica of the Solid State, 56 (2014) 1071-1080
Show abstract ▽
High-porosity samples of beech wood biocarbon (BE-C) were prepared by pyrolysis at carbonization temperatures T carb = 650, 1300, and 1600°C, and their resistivity ρ and thermal conductivity κ were studied in the 5–300 and 80–300 K temperature intervals. The experimental results obtained were evaluated by invoking X-ray diffraction data and information on the temperature dependences ρ(T) and κ(T) for BE-C samples prepared at T carb = 800, 1000, and 2400°C, which were collected by the authors earlier. An analysis of the κ(T carb) behavior led to the conclusion that the samples under study undergo an amorphous-nanocrystalline phase transition in the interval 800°C < T carb < 1000°C. Evaluation of the electronic component of the thermal conductivity revealed that the Lorentz number of the sample prepared at T carb = 2400°C exceeds by far the classical Sommerfeld value, which is characteristic of metals and highly degenerate semiconductors.
Mayo, 2014 | DOI: 10.1134/S1063783414050229
Reactividad de Sólidos
High and stable CO2 capture capacity of natural limestone at Ca-looping conditions by heat pretreatment and recarbonation synergy
Valverde, JM; Sanchez-Jimenez, PE; Perez-Maqueda, LAFuel, 123 (2014) 79-85
Show abstract ▽

The Ca-looping (CaL) process, based on the multicyclic carbonation/calcination of limestone derived CaO, has emerged recently as a potentially economically advantageous technology to achieve sustainable postcombustion and precombustion CO2 capture efficiencies. Yet, a drawback that hinders the efficiency of the CaL process is the drastic drop of limestone capture capacity as the number of carbonation/calcination cycles is increased. Precalcination of limestone at high temperatures for a prolonged period of time has been proposed as a potential technique to reactivate the sorbent, which is however precluded by regeneration temperatures above 850 degrees C and low CO2 concentrations in the carbonator to be found in the practical situation. Under these conditions, heat pretreatment leads to a stable yet very small CaO conversion. On the other hand, the introduction of a recarbonation stage between the ordinary carbonation and calcination stages has been shown to decelerate the rate of sorbent activity decay even though this favorable effect is not noticeable up to a number of above 10-15 cycles. The present manuscript demonstrates that the synergetic action of heat pretreatment and recarbonation yields a high and stable value for the multicyclic conversion of limestone derived CaO. It is foreseen that recarbonation of heat pretreated limestone would lead to a reduction of process costs especially in the case of precombustion applications. Even though sorbent purging will always be needed because of ash accumulation and sul-phation in postcombustion CO2 capture applications, the stable and high multicyclic CaO conversion achieved by the combination of these techniques would make it necessary to a lesser extent.
Mayo, 2014 | DOI: 10.1016/j.fuel.2014.01.045
Química de Superficies y Catálisis
Could an efficient WGS catalyst be useful in the CO-PrOx reaction?
Reina, TR; Papadopoulou, E; Palma, S; Ivanova, S; Centeno, MA; Ioannides, T; Odriozola, JAApplied Catalysis B: Environmental, 150-151 (2014) 554-563
Show abstract ▽
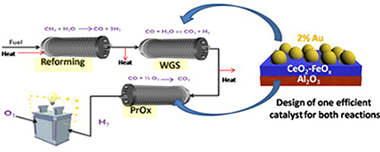
This work presents an evaluation of a high performance series of water gas shift (WGS) catalysts in the preferential CO oxidation reaction (PrOx) in order to examine the applicability of the same catalyst for both processes as a first step for coupling both reactions in a single process. Gold based catalysts are applied in an extensive study of the CO-PrOx reaction parameters, such as λ, WHSV, CO concentration and [H2O]/[CO2] ratio in order to obtain the best activity/selectivity balance. CO and H2 oxidation reactions were treated separately in order to establish the degree of CO/H2 oxidation competition. Additionally the catalysts behavior in the CO-PrOx parallel reactions such a WGS and RWGS have been also carried out to analyze their effect on product composition.
Mayo, 2014 | DOI: 10.1016/j.apcatb.2014.01.001
- ‹ anterior
- 277 of 420
- siguiente ›














