Scientific Papers in SCI
2017
2017
Química de Superficies y Catálisis
Gold catalysts screening in base-free aerobic oxidation of glucose to gluconic acid
Megias-Sayago, C.; Ivanova, S.; Lopez-Cartes, C.; Centeno, M.A.; Odriozola, J.A.Catalysis Today, 279 (2017) 148-154 DOI: 10.1016/j.cattod.2016.06.046
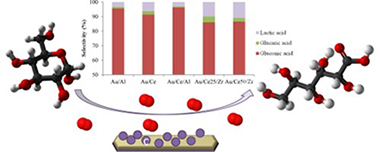
Abstract
Base-free aerobic oxidation of glucose in presence of Au/Al2O3, Au/CeO2, Au/CeO2(20 wt%)/Al2O3, Au/CeO2(25 wt%)/ZrO2 and Au/CeO2(50 wt%)/ZrO2 catalysts using molecular oxygen at atmospheric pressure is studied. Within the whole series high conversion and selectivity to gluconic acid are observed after 18 h of reaction at 120 degrees C. The activity and especially the selectivity changes are related to the support nature in a way that the higher the Lewis acidity of the support the lower the selectivity to gluconic acid and the higher the production of lactic acid. The highest yield to gluconic acid is obtained over Au/Al2O3 for which the influence of the reaction time, temperature and stirring rate are further evaluated and discussed.
January, 2017 · DOI: 10.1016/j.cattod.2016.06.046
2016
2016
Química de Superficies y Catálisis
Selectivity control in oxidation of 1-tetradecanol on supported nano Au catalysts
Martinez-Gonzalez, S; Ivanova, S; Dominguez, MI; Corberan, VCCatalysis Today, 278 (2016) 113-119 DOI: 10.1016/j.cattod.2016.06.019
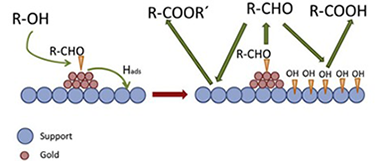
Abstract
Selective oxidation of tetradecanol, a model higher fatty alcohol, on Au/CeO2-Al2O3 catalyst has been investigated to assess the factors that control selectivity. The analysis of the effect of operation conditions (temperature, run time and alcohol/metal (AIM) ratio) on catalytic performance revealed a quite complex reaction network, in which acid formation starts only after a certain level of conversion is reached. This level depends linearly on the total support surface available, indicating that it must be saturated by species generated by the reaction itself to allow acid formation to start. Addition of water to reaction medium did not modify this level, indicating that such species is not adsorbed water, as previously hypothesized, but probably spilled over hydrogen species. The resulting drastic change in the selectivity trends makes the ratio A/M a critical factor to control selectivity to aldehyde and to acid. Selectivity to ester is less sensible to operation parameters. It is noteworthy that aldehyde yields up to 27% with 90% selectivity, and acid yields up to 40% with 81% selectivity can be reached by proper selection of operation parameters.
December, 2016 · DOI: 10.1016/j.cattod.2016.06.019
Reactividad de Sólidos
The calorimetric analysis as a tool for studying the aging hardening mechanism of a Cu-10wt%Ni-5.5wt%Sn alloy
Dianez, MJ; Donoso, E; Sayagues, MJ; Perejon, A; Sanchez-Jimenez, PE; Perez-Maqueda, LA; Criado, JMJournal of Alloys and Compounds, 688 (2016) 288-294 DOI: 10.1016/j.jallcom.2016.07.021
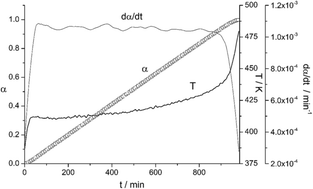
Abstract
The transformations of a Cu-10wt%Ni-5.5wt%Sn alloy as a function of the aging time in the range from room temperature up to 600 degrees C have been followed by Differential Scanning Calorimetry (DSC). The results obtained have shown that this alloy undergone two overlapping exothermic phase transitions with DSC peaks at 208 degrees C and 305 degrees C, respectively, followed by an endothermic phase transformation with a DSC peak at 526 degrees C. The structural analysis by TEM, ED, EDX and XRD of the intermediates phases previously discriminated by DSC suggests that the first exothermic peak is associated to the spinodal decomposition of the sample, while the second one is associated to the segregation of a DO22 (Cu-x-Ni1-x)(3)Sn tetragonal phase coherent with the alpha-Cu structure of the starting alloy. The endothermic peak has been associated to the precipitation of cubic DO3 nanocrystals from the DO22 phase previously formed. The microhardness measurements carried out in combination with the structural characterization demonstrate that the aging hardening of the alloy under study is exclusively due to the formation of the coherent DO22 phase. The DO22/DO3 transition leads to a dramatic drop of the hardness of the alloy.
December, 2016 · DOI: 10.1016/j.jallcom.2016.07.021
Nanotecnología en Superficies y Plasma
Stoichiometric Control of SiOx Thin Films Grown by Reactive Magnetron Sputtering at Oblique Angles
Garcia-Valenzuela, A; Alvarez, R; Lopez-Santos, C; Ferrer, FJ; Rico, V; Guillen, E; Alcon-Camas, M; Escobar-Galindo, R; Gonzalez-Elipe, AR; Palmero, APlasma Processes and Polymers, 13 (2016) 1242-1248 DOI: 10.1002/ppap.201600077
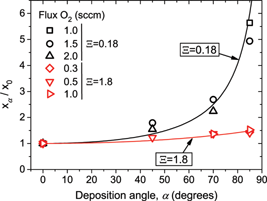
Abstract
The deposition of SiOx (x <= 2) compound thin films by the reactive magnetron sputtering technique at oblique angles is studied from both theoretical and experimental points of view. A simple mathematical formula that links the film stoichiometry and the deposition conditions is deduced. Numerous experiments have been carried out to test this formula at different deposition pressures and oblique angle geometries obtaining a fairly good agreement in all studied conditions. It is found that, at low deposition pressures, the proportion of oxygen with respect to silicon in the film increases a factor of similar to 5 when solely tilting the film substrate with respect to the target, whereas at high pressures the film stoichiometry depends very weakly on the tilt angle. This behavior is explained by considering the fundamental processes mediating the growth of the film by this technique.
December, 2016 · DOI: 10.1002/ppap.201600077
Química de Superficies y Catálisis
Influence of the ionic liquid presence on the selective oxidation of glucose over molybdenum based catalysts
Megias-Sayago, C; Carrasco, CJ; Ivanova, S; Montilla, FJ; Galindo, A; Odriozola, JACatalysis Today, 278 (2016) 82-90 DOI: 10.1016/j.cattod.2016.06.040
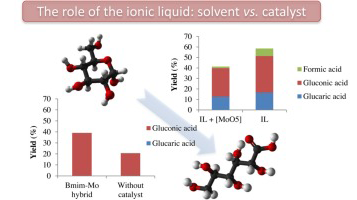
Abstract
Two different approaches are proposed in this work in order to study the influence of the ionic liquid presence in the reaction of glucose oxidation by H2O2 in mild conditions. The ionic liquids are applied either as a solvent by using homogeneous Mo based catalyst, [Mo(O)(O2)2(H2O)n] complex, or by using it as an integral part of a heterogeneous catalyst, organic inorganic hybrids based on Mo Keggin structure. Both catalytic strategies resulted in acceptable glucose transformation degrees but lead to different oxidation products depending on the role of the ionic liquid. The hybrid approach restrains the number of the received products being the most selective one. A detailed study of the effect of the hybrid nature and reaction conditions is proposed in the second part of this study.
December, 2016 · DOI: 10.1016/j.cattod.2016.06.040
Materiales Ópticos Multifuncionales
Three-Dimensional Optical Tomography and Correlated Elemental Analysis of Hybrid Perovskite Microstructures: An Insight into Defect-Related Lattice Distortion and Photoinduced Ion Migration
Galisteo-Lopez, JF; Li, YL; Miguez, HJournal of Physical Chemistry Letters, 7 (2016) 5227-5234 DOI: 10.1021/acsjpclett.6b02456
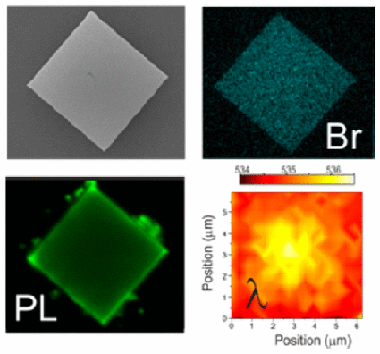
Abstract
Organic lead halide perovskites have recently been proposed for applications in light-emitting devices of different sorts. More specifically, regular crystalline microstructures constitute an efficient light source and fulfill the geometrical requirements to act as resonators, giving rise to waveguiding and optical amplification. Herein we show three-dimensional laser scanning confocal tomography studies of different types of methylammonium lead bromide microstructures which have allowed us to dissect their photoemission properties with a precision of 0.036 mu m(3). This analysis shows that their spectral emission presents strong spatial variations which can be attributed to defect-related lattice distortions. It is also largely enhanced under light exposure, which triggers the migration of halide ions away from illuminated regions, eventually leading to a strongly anisotropic degradation. Our work points to the need for performing an optical quality test of individual crystallites prior to their use in optoelectronics devices and provides a means to do so.
December, 2016 · DOI: 10.1021/acsjpclett.6b02456
Nanotecnología en Superficies y Plasma
Non-Enzymatic Glucose Sensors Based on Nickel Nanoporous Thin Films Prepared by Physical Vapor Deposition at Oblique Angles for Beverage Industry Applications
Salazar, P; Rico, V; Gonzalez-Elipe, ARJournal of the Electrochemical Society, 163 (14) (2016) B704-B709 DOI: 10.1149/2.1241614jes
Abstract
Nickel nanoporous thin films deposited on Indium tin oxide conductive plates have been prepared by physical vapor deposition in an oblique angle configuration. The scanning electron microscopy characterization of these films revealed a microstructure formed by tilted nanocolumns of ca. 40-60 nm of diameter inclined by ca. 26 degrees with respect to the normal. These highly porous films had ca. 30% of void space and provided a large exposed area and outstanding diffusion properties for sensor applications. X-ray diffraction analysis confirmed the deposition of metallic nickel, while Raman and X-ray photoelectron spectroscopies demonstrated that electrochemically treated films presented an oxi/hydroxide outer layer that is the active phase for glucose sensing. The activated electrodes had a high sensitivity (2.05 A M-1 cm(-2)), an excellent coefficient of determination (R-2: 0.999), an outstanding reproducibility (3.2%) and a detection limit of 0.34 mu M. Their glucose selectivity was excellent with regard to common electroactive interferences and other sugars found in agro-alimentary products. Tests carried out with commercial beverages proved the reliability of these electrodes for glucose analysis in real conditions.
December, 2016 · DOI: 10.1149/2.1241614jes
Materiales de Diseño para la Energía y Medioambiente
Microstructure, elastic, and inelastic properties of biomorphic carbons carbonized using a Fe-containing catalyst
Orlova, TS; Kardashev, BK; Smirnov, BI; Gutierrez-Pardo, A; Ramirez-Rico, JPhysics of the Solid State, 58 (2016) 2481-2487 DOI: 10.1134/S1063783416120234
Abstract
The microstructure and amplitude dependences of the Young’s modulus E and internal friction (logarithmic decrement δ), and microplastic properties of biocarbon matrices BE-C(Fe) obtained by beech tree carbonization at temperatures Tcarb = 850–1600°C in the presence of an iron-containing catalyst are studied. By X-ray diffraction analysis and transmission electron microscopy, it is shown that the use of Fe-catalyst during carbonization with Tcarb ≥ 1000°C leads to the appearance of a bulk graphite phase in the form of nanoscale bulk graphite inclusions in a quasi-amorphous matrix, whose volume fraction and size increase with Tcarb. The correlation of the obtained dependences E(Тcarb) and δ(Tcarb) with microstructure evolution with increasing Тcarb is revealed. It is found that E is mainly defined by a crystalline phase fraction in the amorphous matrix, i.e., a nanocrystalline phase at Тcarb < 1150°C and a bulk graphite phase at Tcarb > 1300°C. Maximum values E = 10–12 GPa are achieved for samples with Тcarb ≈ 1150 and 1600°C. It is shown that the microplasticity manifest itself only in biocarbons with Tcarb ≥ 1300°C (upon reaching a significant volume of the graphite phase); in this case, the conditional microyield stress decreases with increasing total volume of introduced mesoporosity (free surface area).
December, 2016 · DOI: 10.1134/S1063783416120234
Química de Superficies y Catálisis
Intensifying glycerol steam reforming on a monolith catalyst: A reaction kinetic model
Bobadilla, LF; Blay, V; Alvarez, A; Dominguez, MI; Romero-Sarria, F; Centeno, MA; Odriozola, JAChemical Engineering Journal, 306 (2016) 933–941 DOI: 10.1016/j.cej.2016.08.021
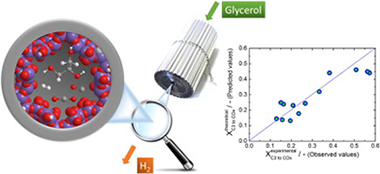
Abstract
In this work, a structured monolithic catalyst has been tested under a wide range of conditions (partial pressure, residence time, temperature and time-on-stream), with the aim of modeling its kinetic behavior and assessing its economic and upscaling potential. We have developed a sequential model to help us interpret both main trends and salient features. Unexpected behavior was found for certain parameter values, which led us to consider kinetic parasitic effects such as mass or heat transfer limitations. By independently invoking these effects, a conciliatory view of the results observed could not be reached. A combined explanation may prove successful, although overfitting could not be ruled out at this point. More importantly, however, the observed salient features of this stable and selective monolith catalyst may hold potential for process intensification of glycerol steam reforming, thus contributing to a more sustainable industry.
December, 2016 · DOI: 10.1016/j.cej.2016.08.021
Reactividad de Sólidos
Influence of Ball Milling on CaO Crystal Growth During Limestone and Dolomite Calcination: Effect on CO2 Capture at Calcium Looping Conditions
Sanchez-Jimenez, PE; Valverde, JM; Perejon, A; de la Calle, A; Medina, S; Perez-Maqueda, LACryst. Growth Des., 16 (2016) 7025–7036 DOI: 10.1021/acs.cgd.6b01228
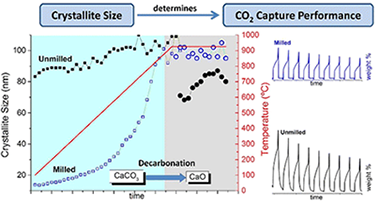
Abstract
Under Calcium-Looping “realistic” operation conditions (high CO2 concentration and temperature), the multicycle CO2 capture capacity performance of CaO derived from limestone and dolomite is inversely related to its crystallite size. Ball-milling the raw sorbents results in the formation of larger nascent CaO nanocrystals during the calcination. Constraint sintering effect due to inert compounds such as dolomitic MgO mitigates the inactivation.
November, 2016 · DOI: 10.1021/acs.cgd.6b01228
Química de Superficies y Catálisis
Recycling of construction and demolition waste generated by building infrastructure for the production of glassy materials
Dominguez, A; Dominguez, MI; Ivanova, S; Centeno, MA; Odriozola, JACeramics International, 42 (2016) 17217-175223 DOI: 10.1016/j.ceramint.2016.06.157
Abstract
The use of waste materials generated by construction and demolition industry to yield valuable glassy materials, i.e. enamel for glazed ceramic tiles and cellular glasses is presented in this study. Both types of materials are produced by one-step treatment at moderate temperatures after simple waste chemical composition adjust. The enamels are manufactured directly from the initial waste powder by melting, while the expanded materials result from mixing of the vitreous material obtained after waste vitrification with an adequate foaming agent and posterior thermal treatment. Through the manuscript the feasibility of one step production of second generation profit materials is discussed in order to help achieving sustainable development and environmental protection.
November, 2016 · DOI: 10.1016/j.ceramint.2016.06.157
Materiales Avanzados
Surface functionalizing of a lipid nanosystem to promote brain targeting: step-by-step design and physico-chemical characterization
Cozar-Bernal, MJ; Garcia-Esteban, E; Sanchez-Soto, PJ; Rabasco, AM; Gonzalez-Rodriguez, MLPharmaceutical Development and Technology, 21 (2016) 823-831 DOI: 10.3109/10837450.2015.1063651
Abstract
The use of lipid nanosystems as drug delivery to the central nervous system may be advantageous over the current strategies. The aim of this study was to develop and characterize functionalized liposomes for treatment of brain diseases. The covalent method of coupling IgG to liposomes via the derivatized lipid 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidophenyl)butyramide](MPB-PE) was investigated. Optimized coupling conditions are shown to result in the efficient conjugation of IgG to liposomes containing low concentrations of MPB-PE (3/1 SH:IgG). The qualitative analysis has shown that after the extrusion process, more homogeneous populations of vesicles have been obtained with a nanometric size suitable to be effective to further anchor the protein. Negative values of zeta potential demonstrate that they are stable systems. Lyophilization was used to maintain the stability of the formulation. These very interesting results encourage further investigations to formulate peptide- and protein-loaded immunoliposomes, making targeting of liposomes as an attractive approach for brain drug delivery.
November, 2016 · DOI: 10.3109/10837450.2015.1063651
Química de Superficies y Catálisis
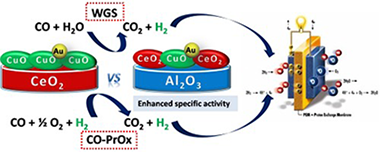
WGS and CO-PrOx reactions using gold promoted copper-ceria catalysts: "Bulk CuO-CeO2 vs. CuO-CeO2/Al2O3 with low mixed oxide content"
Reina, TR; Ivanova, S; Laguna, OH; Centeno, MA; Odriozola, JAApplied Catalysis B-Environmental, 197 (2016) 62-72 DOI: 10.1016/j.apcatb.2016.03.022
Abstract
A copper-ceria bulk catalyst has been compared to a series of catalysts designed according to the as called "supported approach", corresponding to the dispersion of low content mixed copper-ceria oxide on alumina matrix. The principal characteristics of both types of catalysts are contemplated and the differences in their electronic and redox properties discussed in details. As a plus, the gold metal promotion of the catalysts is also envisaged. The advantages of the systems in the CO clean up reactions, WGS and CO-PrOx are commented. While the WGS activity appears to be ruled especially by the Cu/Ce surface to volume ratio, the CO-PrOx reaction is governed by the CuO loading. Gold addition provides benefits only at the low temperature WGS regime. Very importantly, the supported systems are always superior to the bulk configuration in terms of specific activity, a key factor from the catalyst's design perspective.
November, 2016 · DOI: 10.1016/j.apcatb.2016.03.022
Nanotecnología en Superficies y Plasma - Materiales Nanoestructurados y Microestructura
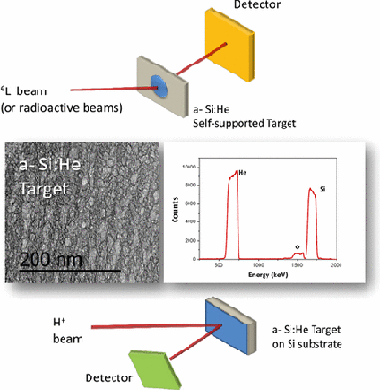
Characterization and Validation of a-Si Magnetron-Sputtered Thin Films as Solid He Targets with High Stability for Nuclear Reactions
Godinho, V; Ferrer, FJ; Fernandez, B; Caballero-Hernandez, J; Gomez-Camacho, J; Fernandez, AACS Omega, 1 (2016) 1229-1238 DOI: 10.1021/acsomega.6b00270
Abstract
In this work, we present our magnetron sputtering based methodology to produce amorphous silicon coatings with closed porosity, as a strategy to fabricate solid helium targets, in the form of supported or self-supported thin films, for nuclear reactions. We show how by changing the He working pressure it is possible to obtain highly porous homogeneous structures incorporating different He amounts. These porous coatings (a-Si: He) are very reproducible from run to run, and the high He amount incorporated makes them excellent candidates for solid He targets. The possibility of producing self-supported films is illustrated here, and its potential use in inverse kinematics experiments with radioactive beams is shown through the dispersion in forward geometry of a stable Li-6 beam. Also the elastic scattering cross-sections for proton from helium were determined using an a-Si: He coating. The results agree well with the ones reported in the literature. These two examples validate our coatings as good candidates to be used as solid He targets in nuclear reactions. The stability of He inside the coatings, fundamental for its use as solid He targets, was investigated, both over time and after irradiation. The coatings proved to be very stable, and the amount of He inside the pores remains unaltered at least 2 years after deposition and after high irradiation fluence (5 x 10(17) particles/cm(2); with a dose rate of 5 x 10(12) particles/(cm(2) s)).
November, 2016 · DOI: 10.1021/acsomega.6b00270
Materiales de Diseño para la Energía y Medioambiente
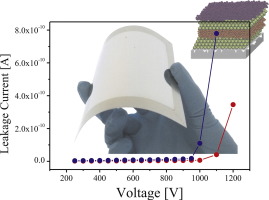
Enhancement of dielectric barrier layer properties by sol-gel and PECVD stacks
Lopez-Lopez, C; Menendez, MF; Menendez, LA; Menendez, A; Sanchez, P; Alba, MD; Sanchez-Cortezon, E; Delgado-Sanchez, JMSurface and Coatings Technology, 305 (2016) 36-40 DOI: 10.1016/j.surfcoat.2016.07.085
Abstract
Thin-film PV modules grown on flexible, light weight, thermally stable and low cost substrates such as stainless steel foil, are an attractive product for solar market applications. When metal foils are used as substrate, it is essential to deposit a dielectric barrier layer to isolate electrically and chemically the thin-film solar cells from the substrate. In this work, SiOx stacks deposited on ‘rough’ stainless steel by a combination of a new sol-gel formulation and a Plasma Enhanced Chemical Vapor Deposition (PECVD) deposition step are reported as a suitable dielectric barrier layer candidate. Using these SiOx multilayers, a smooth and homogeneous film was achieved. X-ray diffraction (XRD) analysis showed that back contact of the solar cell (based on Molybdenum) is not affected by the presence of the barrier layer. Moreover, according to X-ray photoelectron spectroscopy (XPS) and Secondary Ion Mass Spectrometry (SIMS) measurements, this approach led to excellent barrier layer properties against the diffusion of impurities from the stainless steel. A complete electrical characterization of these dielectric barrier layers was also carried out showing good electrical insulation.
November, 2016 · DOI: 10.1016/j.surfcoat.2016.07.085
Materiales de Diseño para la Energía y Medioambiente
Manganese Dioxide Supported on Porous Biomorphic Carbons as Hybrid Materials for Energy Storage Devices
Gutierrez-Pardo, A; Lacroix, B; Martinez-Fernandez, J; Ramirez-Rico, JACS Applied Materials & Interfaces, 8 (2016) 30890-30898 DOI: 10.1021/acsami.6b09361

Abstract
A facile and low-cost method has been employed to fabricate MnO2/C hybrid materials for use as binder-free electrodes for supercapacitor applications. Biocarbon monoliths were obtained through pyrolysis of beech wood, replicating the microstructure of the cellulosic precursor, and serve as 3D porous and conductive scaffolds for the direct growth of MnO, nanosheets by a solution method. Evaluation of the experimental results indicates that a homogeneous and uniform composite material made of a carbon matrix exhibiting ordered hierarchical porosity and MnO, nanosheets with a layered nanocrystalline structure is obtained. The tuning of the MnO2 content and crystallite size via the concentration of KMnO4 used as impregnation solution allows to obtain composites that exhibit enhanced electrochemical behavior, achieving a capacitance of 592 F g(-1) in electrodes containing 3 wt % MnO2 with an excellent cyclic stability. The electrode materials were characterized before and after electrochemical testing.
November, 2016 · DOI: 10.1021/acsami.6b09361
Materiales de Diseño para la Energía y Medioambiente
Thermal conductivity of porous biomorphic SiC derived from wood precursors
Gomez-Martin, A; Orihuela, MP; Ramirez-Rico, J; Chacartegui, R; Martinez-Fernandez, JCeramics International, 42 (2016) 16220-16229 DOI: 10.1016/j.ceramint.2016.07.151
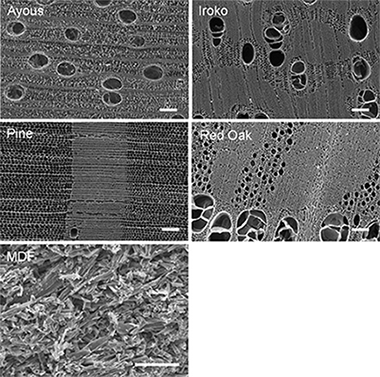
Abstract
Biomorphic SiC is a SiC/Si composite made by the reactive infiltration of molten silicon by capillarity into a carbon preform from high-temperature pyrolysis of a wood porous precursor. When excess silicon is removed, a hierarchically porous SiC material with highly interconnected porosity is obtained. By choosing different wood precursors, different pore size distributions can be obtained thus tailoring the resulting properties.
We study the thermal conductivity of porous biomorphic SiC from five different precursors, including a recycled wood product, in order to determine the microstructure-conductivity correlation. Here, we remove the excess of silicon by a high temperature capillary extraction-evaporation method. We used the laser flash technique to measure thermal diffusivity in the range similar to 300 K to 973 K, in order to determine the thermal conductivity. Thermal conductivities in the range 4-88 W/m K were achieved. The temperature, porosity, pore shape and orientation, and the treatment used to remove the remaining silicon all have a significant impact in the resulting thermal conductivity. We explain this influence in a first approximation in terms of a geometrical model.
November, 2016 · DOI: 10.1016/j.ceramint.2016.07.151
An innovative combination of non-invasive UV–Visible-FORS, XRD and XRF techniques to study Roman wall paintings from Seville, Spain
Garofano, Isabel; Luis Perez-Rodriguez, Jose; Dolores Robador, Maria; Duran, AdrianJournal of Cultural Heritage, 22 (2016) 1028-1039 DOI: 10.1016/j.culher.2016.07.002
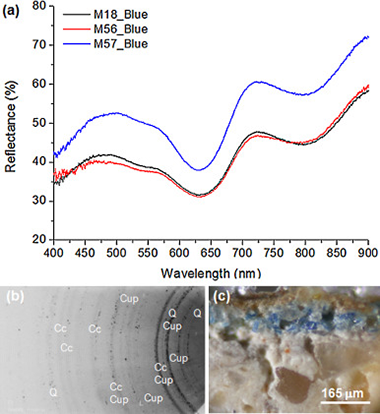
Abstract
This study attempts to establish the advantages and limitations of the combined use of portable UV–Vis-FORS and XRF-XRD portable equipment for the non-invasive characterisation of pigments from Roman wall paintings from Seville, Spain, dated to the first and second century AD. XRD revealed the presence of calcite, dolomite and aragonite, indicating the colour white. Egyptian blue was identified using FORS and XRF, and additional information was obtained with XRD. For the colour green, FORS and mainly FTIR and colorimetry enabled the distinction between glauconite and celadonite, although other techniques were necessary to classify all components of the green areas by determining the presence of cuprorivaite, chlorite and chromium. For the colours yellow and red, the presence of goethite, yellow ochre, cinnabar and haematite was confirmed using FORS and XRF in some cases; the results were corroborated by XRD. Chromatic characterisation and the values of inflection points of FORS spectra enabled a better differentiation between reddish colours (orange, brown, purple and pink). The XRD and XRF techniques revealed that violet was created by mixing red haematite and Egyptian blue and slight variations in FORS spectra confirmed this.
November, 2016 · DOI: 10.1016/j.culher.2016.07.002
Materiales Coloidales
Multifunctional Eu-doped NaGd(MoO4)(2) nanoparticles functionalized with poly(L-lysine) for optical and MRI imaging
M. Laguna; N.O. Nuñez; V. Rodríguez; E. Cantelar, G. Stepien, M.L. García, J.M. de la Fuente; M. OcañaDalton Transactions, 45 (2016) 16354-16365 DOI: 10.1039/c6dt02663j
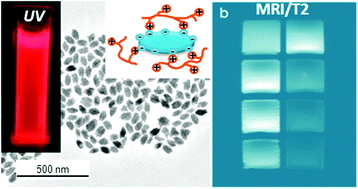
Abstract
A method for the synthesis of non-aggregated and highly uniform Eu3+ doped NaGd(MoO4)(2) nanoparticles is reported for the first time. The obtained particles present tetragonal structure, ellipsoidal shape and their size can be varied by adjusting the experimental synthesis parameters. These nanoparticles, which were coated with citrate anions and functionalised with PLL, have also been developed in order to improve their colloidal stability in physiological medium (2-(N-morpholino) ethanesulfonic acid, MES). A study of the luminescent dynamics of the samples as a function of the Eu doping level has been conducted in order to find the optimum nanophosphors, whose magnetic relaxivity and cell viability have also been evaluated for the first time for this system, in order to assess their suitability as multifunctional probes for optical (in vitro) and magnetic bioimaging applications.
November, 2016 · DOI: 10.1039/c6dt02663j
Nanotecnología en Superficies y Plasma - Materiales Nanoestructurados y Microestructura
Tailor-made preparation of Co-C, Co-B, and Co catalytic thin films using magnetron sputtering: insights into structure-composition and activation effects for catalyzed NaBH4 hydrolysis
Paladini, M; Godinho, V; Arzac, GM; de Haro, MCJ; Beltran, AM; Fernandez, ARSC Advances, 6 (2016) 108611-108620 DOI: 10.1039/c6ra23171c
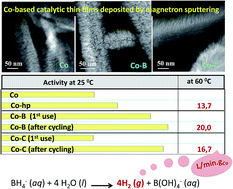
Abstract
The magnetron sputtering (MS) methodology is a powerful tool for tailor-made fabrication of Co-based thin film catalysts with controlled microstructures and compositions for sodium borohydride (SBH) hydrolysis. In particular, Co-C catalysts were tested in this reaction and compared to Co-B and Co catalyst coatings. The microstructural and chemical analyses by X-ray diffraction (XRD), scanning and transmission electron microscopy (SEM and TEM), Rutherford back scattering (RBS) and X-ray photoelectron spectroscopy (XPS) were used to characterize a complete library of thin film catalysts. Pure Co materials were characterized by their nanocrystalline microstructure, and grain refinement was achieved via an increase in the deposition pressure. The incorporation of boron or carbon via co-deposition results in amorphization and dispersion of the active metallic Co phase. The composition can be tuned while keeping a controlled microstructure, and a comparison of activity at 25 degrees C was performed on catalysts deposited on Ni foam substrates. A comparison of the initial activities showed that the Co-B samples were more active than the Co-C samples because of electronic effects. However, a strong activation was found for the Co-C catalysts after the first use. This effect was dependent upon the incorporation of cobalt boride (CoxB) species on the catalysts' surface, as shown by XPS. After the first several uses, the activity of the Co-C samples (values up to 2495 mL min(-1) g(catalyst)(-1)) were as high as that of fresh Co-B, and the surface composition of both the catalysts was similar. This activation was not observed for the pure Co and was very weak for the Co-B catalysts. The use of polymeric (PTFE) substrates (flexible membranes) illustrated the versatility of the methodology to obtain catalytic membranes and allowed for a TEM microstructural analysis at the nanoscale. Catalytic activities at 60 degrees C were as high as 16.7 and 20 L min(-1) g(Co)(-1) for the Co-C and Co-B membranes, respectively. We determined the optimized conditions to increase the catalytic activity of Co-based coatings prepared via magnetron sputtering.
November, 2016 · DOI: 10.1039/c6ra23171c
Materiales Coloidales
Optical sensing by integration of analyte-sensitive fluorophore to particles
Carrillo-Carrion, C; Escudero, A; Parak, WJTrAC Trends in Analytical Chemistry, 84 (2016) 84-85 DOI: 10.1016/j.trac.2016.05.001
Abstract
Analyte-sensitive fluorophores are a common tool in analytical chemistry. In case they are conjugated to the surface of colloidal nanoparticles new or improved applications are possible. An overview of the potential of such fluorophore-particle conjugates is given by means of several examples. First, using pH-sensitive fluorophores attached to particles are a helpful tool for investigating particle uptake by cells, as they can indicate whether particles are in the neutral slightly alkaline extracellular medium, or in acidic intracellular vesicles after endocytosis. Second, relating to lifetime-based methodologies, the fluorescence resonance energy transfer between fluorophores attached to quantum dots leads to longer lifetimes, improving their performance and expanding the possibilities of methods, such as lifetime imaging for in vivo applications. It also can be exploited for multiplexing approaches, in which the effective lifetime of the fluorophores can be tuned, allowing thus for the detection of several analytes based on temporal discrimination. Attention is focused to these three areas of application, because they are among the most reported in recent literature, and therefore of particular interest.
November, 2016 · DOI: 10.1016/j.trac.2016.05.001
Reactividad de Sólidos
Combined TGA-MS kinetic analysis of multistep processes. Thermal decomposition and ceramification of polysilazane and polysiloxane preceramic polymers
Garcia-Garrido, C; Sanchez-Jimenez, PE; Perez-Maqueda, LA; Perejon, A; Criado, JMPhysical Chemistry Chemical Physics, 18 (2016) 29348-29360 DOI: 10.1039/c6cp03677e
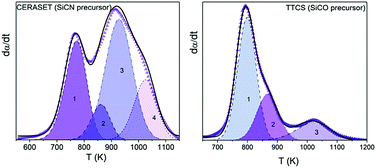
Abstract
The polymer-to-ceramic transformation kinetics of two widely employed ceramic precursors, 1,3,5,7-tetramethyl-1,3,5,7-tetravinylcyclotetrasiloxane (TTCS) and polyureamethylvinylsilazane (CERASET), have been investigated using coupled thermogravimetry and mass spectrometry (TG-MS), Raman, XRD and FTIR. The thermally induced decomposition of the pre-ceramic polymer is the critical step in the synthesis of polymer derived ceramics (PDCs) and accurate kinetic modeling is key to attaining a complete understanding of the underlying process and to attempt any behavior predictions. However, obtaining a precise kinetic description of processes of such complexity, consisting of several largely overlapping physico-chemical processes comprising the cleavage of the starting polymeric network and the release of organic moieties, is extremely difficult. Here, by using the evolved gases detected by MS as a guide it has been possible to determine the number of steps that compose the overall process, which was subsequently resolved using a semiempirical deconvolution method based on the Frasier-Suzuki function. Such a function is more appropriate that the more usual Gaussian or Lorentzian functions since it takes into account the intrinsic asymmetry of kinetic curves. Then, the kinetic parameters of each constituent step were independently determined using both model-free and model-fitting procedures, and it was found that the processes obey mostly diffusion models which can be attributed to the diffusion of the released gases through the solid matrix. The validity of the obtained kinetic parameters was tested not only by the successful reconstruction of the original experimental curves, but also by predicting the kinetic curves of the overall processes yielded by different thermal schedules and by a mixed TTCS-CERASET precursor.
November, 2016 · DOI: 10.1039/c6cp03677e
Materiales Avanzados
Surface functionalization of a lipid nanosystem to promote brain targeting: step-by-step design and physico-chemical characterization
Cózar-Bernal, MJ; García-Esteban, E; Sánchez-Soto, PJ; Rabasco, AM; González-Rodríguez, MLPharmaceutical Development and Technology, 21 (7) (2016) 823-831 DOI: 10.3109/10837450.2015.1063651
Abstract
The use of lipid nanosystems as drug delivery to the central nervous system may be advantageous over the current strategies. The aim of this study was to develop and characterize functionalized liposomes for treatment of brain diseases. The covalent method of coupling IgG to liposomes via the derivatized lipid 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidophenyl)butyramide](MPB-PE) was investigated. Optimized coupling conditions are shown to result in the efficient conjugation of IgG to liposomes containing low concentrations of MPB-PE (3/1 SH:IgG). The qualitative analysis has shown that after the extrusion process, more homogeneous populations of vesicles have been obtained with a nanometric size suitable to be effective to further anchor the protein. Negative values of zeta potential demonstrate that they are stable systems. Lyophilization was used to maintain the stability of the formulation. These very interesting results encourage further investigations to formulate peptide- and protein-loaded immunoliposomes, making targeting of liposomes as an attractive approach for brain drug delivery.
November, 2016 · DOI: 10.3109/10837450.2015.1063651
Glutamate microbiosensors based on Prussian Blue modified carbon fiber electrodes for neuroscience applications: In-vitro characterization
Salazar, P; Martin, M; O'Neill, RD; Gonzalez-Mora, JLSensors and Actuators B: Chemical, 235 (2016) 117-125 DOI: 10.1016/j.snb.2016.05.057
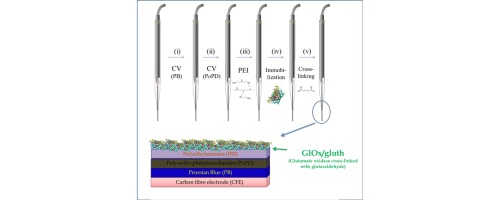
Abstract
Herein we report a Prussian Blue modified carbon fiber electrode (CFE/PB) to be used in microbiosensors for glutamate monitoring in physiological applications as an alternative to the classical Pt and Pt-Ir transducers. Their low dimensions (∼250 μm CFE length and ∼10 μm diameter) are advantageous for measuring in living tissues. In addition, PB-modified microelectrodes allow the detection of enzyme-generated hydrogen peroxide at a low applied potential (∼0.0 V against SCE), contrasting the high potential used in many previous designs (∼0.7 V), decreasing the endogenous interference contributions. Moreover, the electrosynthesized polymer, poly-o-phenylenediamine (PoPD), was used to improve biosensor stability and selectivity. CFE/PB was conveniently characterized using impedance, Raman and XPS spectroscopies. Optimization of the fabrication procedure and analytical conditions is described, including activation of CFE/PB, enzyme enrichment, cross-linking, stabilization and anti-interference. A range of analytical parameters were also characterized such as sensitivity, limit of detection, linear range, and enzymatic loading. Finally, an optimized biosensor displaying a linear sensitivity of 135 ± 2 nA μM−1 cm−2 (n = 3), LOD of <2 μM, linear range up to 150 μM and effectively free of interference, is proposed as a suitable candidate for in-vivo glutamate monitoring in the central nervous system.
November, 2016 · DOI: 10.1016/j.snb.2016.05.057
Reactividad de Sólidos
Magnesium hydride for energy storage applications: The kinetics of dehydrogenation under different working conditions
Perejon, A; Sanchez-Jimenez, PE; Criado, JM; Perez-Maqueda, LAJournal of Alloys and Compounds, 681 (2016) 571-579 DOI: 10.1016/j.jallcom.2016.04.191
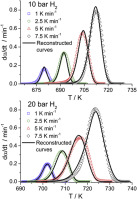
Abstract
A new approach to the kinetics of magnesium hydride dehydrogenation is considered. A model able to predict the dehydrogenation under different experimental conditions has been proposed. A new combined kinetic analysis method, which considers the thermodynamic of the process according to the microreversibility principle, has been used for performing the kinetic analysis of data obtained under different thermal schedules at hydrogen pressures ranging from high vacuum up to 20 bar.
The kinetic analysis shows that the dehydrogenation mechanism of magnesium hydride depends on the experimental conditions. Thus, the reaction follows a first order kinetics, equivalent to an Avarmi-Erofeev kinetic model with an Avrami coefficient equal to 1, when carried out under high vacuum, while a mechanism of tridimensional growth of nuclei previously formed (A3) is followed under hydrogen pressure. An explanation of the change of mechanism is given. It has been shown that the activation energy is closed to the Mg-H bond breaking energy independently of the hydrogen pressure surrounding the sample, which suggests that the breaking of this bond would be the rate limiting step of the process. The reliability of the calculated kinetic parameters is tested by comparing simulated and experimental curves.
October, 2016 · DOI: 10.1016/j.jallcom.2016.04.191
Materiales Coloidales
Quantitative uptake of colloidal particles by cell cultures
Feliu, N; Huhn, J; Zyuzin, MV; Ashraf, S; Valdeperez, D; Masood, A; Said, AH; Escudero, A; Pelaz, B; Gonzalez, E; Duarte, MAC; Roy, S; Chakraborty, I; Lim, ML; Sjoqvist, S; Jungebluth, P; Parak, WJScience of the Total Environment, 568 (2016) 819-828 DOI: 10.1016/j.scitotenv.2016.05.213
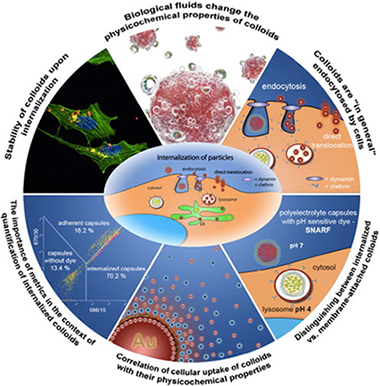
Abstract
The use of nanotechnologies involving nano-and microparticles has increased tremendously in the recent past. There are various beneficial characteristics that make particles attractive for a wide range of technologies. However, colloidal particles on the other hand can potentially be harmful for humans and environment. Today, complete understanding of the interaction of colloidal particles with biological systems still remains a challenge. Indeed, their uptake, effects, and final cell cycle including their life span fate and degradation in biological systems are not fully understood. This is mainly due to the complexity of multiple parameters which need to be taken in consideration to perform the nanosafety research. Therefore, we will provide an overview of the common denominators and ideas to achieve universal metrics to assess their safety. The review discusses aspects including how biological media could change the physicochemical properties of colloids, how colloids are endocytosed by cells, how to distinguish between internalized versus membrane-attached colloids, possible correlation of cellular uptake of colloids with their physicochemical properties, and how the colloidal stability of colloids may vary upon cell internalization. In conclusion three main statements are given. First, in typically exposure scenarios only part of the colloids associated with cells are internalized while a significant part remain outside cells attached to their membrane. For quantitative uptake studies false positive counts in the form of only adherent but not internalized colloids have to be avoided. pH sensitive fluorophores attached to the colloids, which can discriminate between acidic endosomal/lysosomal and neutral extracellular environment around colloids offer a possible solution. Second, the metrics selected for uptake studies is of utmost importance. Counting the internalized colloids by number or by volume may lead to significantly different results. Third, colloids may change their physicochemical properties along their life cycle, and appropriate characterization is required during the different stages.
October, 2016 · DOI: 10.1016/j.scitotenv.2016.05.213
Química de Superficies y Catálisis
Forced deactivation and postmortem characterization of a metallic microchannel reactor employed for the preferential oxidation of CO (PROX)
Laguna, OH; Dominguez, MI; Centeno, MA; Odriozola, JAChemical Engineering Journal, 302 (2016) 650-662 DOI: 10.1016/j.cej.2016.05.104
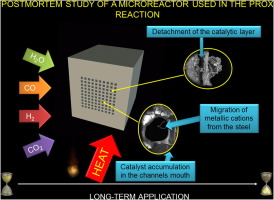
Abstract
This manuscript is one of the few works presenting evidences of the effect of prolonged use of a microreactor. Our reactor has been designed for the PROX reaction. Near to 550 h of operation under different feed-streams, including CO2 and H2O, in the 100-300 degrees C temperature range, and several regeneration cycles, and a final forced deactivation during similar to 360 h resulted in the permanent loss of activity of the microreactor. This could be attributed to some phenomena whose have compromised the chemical nature of the catalyst and that of the reactor including: displacement of the coating to the mouth of the channels, detachments and cracks of the catalytic layer, migration of some elements of the metallic substrate to the surface (Fe, Cr, Y), and deposition of carbonaceous species from the reaction over the catalytic layer and/or the metallic substrate. Furthermore, sulfur compounds were detected in both inlet and outlet zones of the microreactor, coming probably from a lubricant applied over the screws that sealed the assembling of the microreactor.
This is a first approach for understanding possible effects of deactivation during long-term applications of a microreactor in the PROX reaction that could be considered as a case study useful for future designs of this kind of devices. The presented information could be extrapolated to similar reactions where thermal treatments along with highly corrosive atmospheres would be applied, in order to carry out a more appropriate design of future generations" of microreactors, with a longer useful life. For that purpose not only the adequate selection of the catalysts must be done, but also the adequate choice of the fabrication material of the reactors is needed.
October, 2016 · DOI: 10.1016/j.cej.2016.05.104
Reactividad de Sólidos
Enhanced carbon nanotube dispersion in 3YTZP/SWNTs composites and its effect on room temperature mechanical and electrical properties
Gallardo-Lopez, A; Morales-Rodriguez, A; Vega-Padillo, J; Poyato, R; Munoz, A; Dominguez-Rodriguez, AJournal of Alloys and Compounds, 682 (2016) 70-79 DOI: 10.1016/j.jallcom.2016.04.262
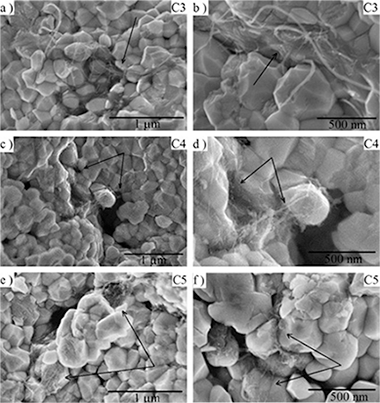
Abstract
In this work, several modifications of the colloidal processing technique and spark plasma sintering (SPS) to prepare yttria tetragonal zirconia composites (YTZP) with single walled carbon nanotubes (SWNT) have been tested with the aim of eliminating SWNT agglomerates. These modifications include high versus low energy ultrasonic agitation during colloidal processing, lyophilization of the 3YTZP/SWNT slurry and electrical insulation during sintering of the composites. Semi-quantitative microstructural characterization of the carbon nanotube distribution in the sintered composites showed that high energy ultrasonic agitation reduces drastically agglomerate size. Lyophilization of the mixed suspensions avoids SWNT bundle size growth. Combination of both produces an enhanced carbon nanotube network distribution along the grain boundaries (GB) due to the absence of carbon nanotube agglomerates and to a limited SWNT bundle size. This results in an increase of the real SWNT content in the GBs up to nominal SWNT content and therefore an enhanced SWNT efficiency in the composites. The agglomerate-free highly-dispersed composites exhibit a decrease in density together with grain size refinement, a decrease in room temperature hardness, an increase in flexural strength and a most significant increase in room temperature electrical conductivity. Improved SWNT distribution also lowers electrical percolation threshold to a very low level in SWNT ceramic composites, <1 vol% SWNT.
October, 2016 · DOI: 10.1016/j.jallcom.2016.04.262
Química de Superficies y Catálisis
Liquid-phase oxidation with hydrogen peroxide of benzyl alcohol and xylenes on Ca-10(PO4)(6)(OH)(2) - CaWO4
Dominguez, MI; Cojocaru, B; Tudorache, M; Odriozola, JA; Centeno, MA; Parvulescu, VIComptes Rendus Chimie, 19 (2016) 1156-1165 DOI: 10.1016/j.crci.2015.10.013
Abstract
A W-containing apatite (W/HAp) catalyst was prepared following a hydrothermal synthesis route and served as a model catalyst. Crystallographic analysis indicated that the resulting material contained hydroxyapatite, Ca10-3xWx(PO4)(6)(OH)(2), W-hydroxyapatite, calcium tungstate, CaWO4, and tricalcium phosphate, Ca-3(PO4)(2). The catalyst was investigated in liquid phase oxidation of benzyl alcohol and xylenes using hydrogen peroxide as an oxidant. For comparison, commercial calcium phosphate, hydroxyapatite and CaWO4 were tested in the same reaction. Calcium phosphate and hydroxyapatite appeared as inactive and decomposed hydrogen peroxide non-selectively. A moderate activity but low hydrogen peroxide efficiency was observed for the CaWO4 phase. In contrast, the W/HAp catalyst showed a reasonable activity and a better hydrogen peroxide efficiency in the oxidation of benzyl alcohol and xylenes. This new W/HAp catalyst showed, after six cycles, losses of the activity below 15% compared to the fresh catalyst with no effect on the selectivity. It is noteworthy that ICP-OES analyses showed no tungsten leaching that is the main advantage of this catalyst.
October, 2016 · DOI: 10.1016/j.crci.2015.10.013
Materiales de Diseño para la Energía y Medioambiente
Permeability and mechanical integrity of porous biomorphic SiC ceramics for application as hot-gas filters
Gomez-Martin, A.; Orihuela, M. P.; Becerra, J. A.; Martinez-Fernandez, J.; Ramirez-Rico, J.Materials & Design, 107 (2016) 450-460 DOI: 10.1016/j.matdes.2016.06.060
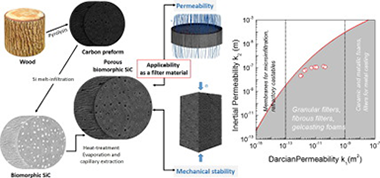
Abstract
Biomorphic SiC is a biotemplated material fabricated by Si melt-infiltration of carbon preforms from wood pyrolysis. In this work, porous bioSiC ceramics from five different wood precursors, with porosities between 45 and 72% were studied for their feasibility in filtering applications.
Gas permeability and mechanical stability were investigated as a function of the microstructure of the starting wood precursor. Air-permeation performance at room temperature was measured for a range of flow rates, and the permeability constants were assessed by fitting of Forchheimer's equation to the experimental data. Darcian permeabilities were achieved in the range 10− 11–10− 12 m2, while inertial terms were in the range 10− 7–10− 8 m, showing a correlation with the average pore size and orientation of the larger channels. Regarding the mechanical stability, maximum compressive strength values were reached in the range of 3–115 MPa.
These results improve our understanding of the ways in which the microstructure influences permeability and mechanical robustness, enabling the device requirements to be tailored by selecting the wood precursor. It was also shown that these materials are promising for hot-gas filtering applications.
October, 2016 · DOI: 10.1016/j.matdes.2016.06.060
Nanotecnología en Superficies y Plasma
High-Rate Deposition of Stoichiometric Compounds by Reactive Magnetron Sputtering at Oblique Angles
Rafael Alvarez, Aurelio Garcia-Valenzuela, Carmen Lopez-Santos, Francisco J. Ferrer, Victor Rico, Elena Guillen, Mercedes Alcon-Camas, Ramon Escobar-Galindo, Agustin R. Gonzalez-Elipe, Alberto PalmeroPlasma Processes and Polymers, 13 (2016) 571-576 DOI: 10.1002/ppap.201600019
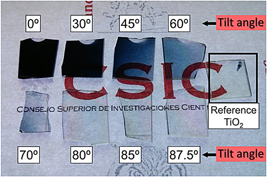
Abstract
Target poisoning in reactive magnetron sputtering deposition of thin films is an undesired phenomenon, well known for causing a drastic fall of the process efficiency. We demonstrate that when this technique is operated at oblique angles, films with composition raging from pure metallic to stoichiometric compound can be grown in non-poisoned conditions, thus avoiding most of the associated drawbacks. We have employed amorphous TiOx, although the presented results can be easily extrapolated to other materials and conditions. It is found that the proposed method improves 400% the growth rate of TiO2 thin films.
October, 2016 · DOI: 10.1002/ppap.201600019
Química de Superficies y Catálisis
Au/CeO2 Catalysts: Structure and CO Oxidation Activity
Centeno, MA; Reina, TR; Ivanova, S; Laguna, OH; Odriozola, JACatalysts, 6 (2016) 158 DOI: 10.3390/catal6100158
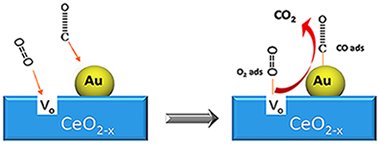
Abstract
In this comprehensive review, the main aspects of using Au/CeO2 catalysts in oxidation reactions are considered. The influence of the preparation methods and synthetic parameters, as well as the characteristics of the ceria support (presence of doping cations, oxygen vacancies concentration, surface area, redox properties, etc.) in the dispersion and chemical state of gold are revised. The proposed review provides a detailed analysis of the literature data concerning the state of the art and the applications of gold–ceria systems in oxidation reactions.
October, 2016 · DOI: 10.3390/catal6100158
Química de Superficies y Catálisis
Au-supported on Fe-doped ceria solids prepared in water-in-oil microemulsions: Catalysts for CO oxidation
Laguna, OH; Centeno, MA; Boutonnet, M; Odriozola, JACatalysis Today, 278 (2016) 140-149 DOI: 10.1016/j.cattod.2016.05.059
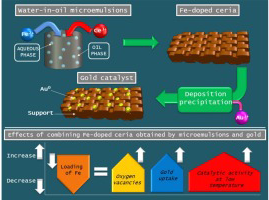
Abstract
Gold catalysts were synthesized by deposition-precipitation employing Fe-doped ceria systems, previously obtained by means of the water-in-oil microemulsions methodology with different iron contents (10, 25 and 50 Fe at.%). The final catalysts were tested in the CO oxidation reaction in presence of H2. After gold deposition the crystalline structure of the supports was not altered. Moreover no XRD lines associated to gold were detected, indicating its high dispersion. Solid solution was generated in all samples, although the segregation of iron oxide was detected for the material with the highest iron loading. This phenomenon was then enhanced for the corresponding gold catalyst that also presented sintering of the gold nanoparticles.
Strong interaction between gold and the oxygen vacancies of the supports was demonstrated, as well as the promotion of the reducibility of surface Ce4+ and Fe3+species at low temperatures. A remarkable promotion of the CO conversion at lower temperatures respect to that of the supports was observed for the gold catalysts. Below 120 °C, lower the amount of iron incorporated, higher the catalytic performance of the catalyst. This behaviour is closely related not only to a high gold dispersion but also to the ability for creating additional oxygen vacancies in the support, required for the CO oxidation reaction.
October, 2016 · DOI: 10.1016/j.cattod.2016.05.059
Nanotecnología en Superficies y Plasma
Cathode and ion-luminescence of Eu:ZnO thin films prepared by reactive magnetron sputtering and plasma decomposition of non-volatile precursors
Gil-Rostra, J; Ferrer, FJ; Martin, IR; Gonzalez-Elipe, AR; Yubero, FJournal of Luminescence, 178 (2016) 139-146 DOI: 10.1016/j.jlumin.2016.01.034
Abstract
This paper reports the luminescent behavior of Eu:ZnO thin films prepared by an one-step procedure that combines reactive magnetron sputtering deposition of ZnO with the plasma activated decomposition of a non-volatile acetylacetonate precursor of Eu sublimated in an effusion cell. Chemical composition and microstructure of the Eu:ZnO thin films have been characterized by several methods and their photo-, cathode- and ion-luminescent properties studied as a function of Eu concentration. The high transparency and well controlled optical properties of the films have demonstrated to be ideal for the development of cathode- and ion- luminescence sensors.
October, 2016 · DOI: 10.1016/j.jlumin.2016.01.034
Materiales Avanzados
Preparation of calcium carbonate as nanoparticles from inorganic precursors and sucrose as additive with potential application as biomaterial
Takabait, F; Mahtout, L; Villarejo, LP; Hurtado, BC; Soto, PJSBoletin de la Sociedad Española de Cerámcia y Vidrio, 55 (2016) 179-184 DOI: 10.1016/j.bsecv.2016.01.006
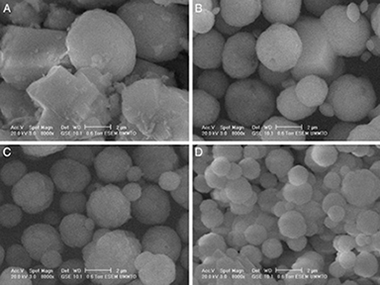
Abstract
En esta comunicación se presentan unos primeros resultados de interés relevante sobre la obtención de carbonato de calcio precipitado como nanopartículas de los polimorfos vaterita y calcita. Se parte de precursores inorgánicos, nitrato de calcio tetrahidratado y bicarbonato de sodio, en presencia de sacarosa empleada como aditivo orgánico en disolución acuosa. Las fases cristalinas formadas se estudian mediante difracción de rayos X con un método cuantitativo y la morfología de las partículas obtenidas, mediante microscopia electrónica de barrido. Cuando no se emplea el aditivo orgánico se consigue la precipitación de calcita, polimorfo más estable termodinámicamente, como fase nanocristalina predominante (83%) mezclada con vaterita. Con una alta concentración del aditivo (67%) se obtiene vaterita como fase mayoritaria (> 98%). La utilización del aditivo en distinta proporción produce la formación de los 2 polimorfos de carbonato de calcio, siendo vaterita la fase predominante. La morfología de las partículas obtenidas muestra la formación de partículas nanoesféricas uniformes con contornos irregulares que se asocian a vaterita, así como partículas romboédricas de calcita cuando está presente, con potencial interés por su biocompatibilidad para su aplicación como biomateriales en implantes óseos.
September, 2016 · DOI: 10.1016/j.bsecv.2016.01.006
Química de Superficies y Catálisis
Growth of carbonaceous nanomaterials over stainless steel foams. Effect of activation temperature
Latorre, N; Cazana, F; Sebastian, V; Royo, C; Romeo, E; Centeno, MA; Monzon, ACatalysis Today, 273 (2016) 41-49 DOI: 10.1016/j.cattod.2016.02.063
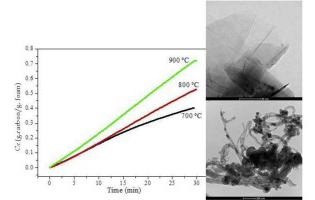
Abstract
Some of the problems that occur during the operation of chemical reactors based of structured catalytic substrates, as monoliths, foams, membranes, cloths, fibres and other systems, are related to the preparation of long term stable coatings. Frequently, the deposition of the catalytic layer is carried out by washcoating, requiring this step a cautious attention, especially in the case of complex geometries, like of that of foams or cloths. In the case of the deposition of layers of carbonaceous materials (CNMs), an alternative route, avoiding the washcoating, it is their direct growth by catalytic decomposition light hydrocarbons (also called CCVD), over the surface of the metallic substrate. In this case, if the metallic substrate is of stainless steel, it already contains the catalytic active phases like Fe and Ni.
In order to optimize the process of CNMs growth over structured metallic substrates, we are studying the effect of the main operational variables of the ethane decomposition reaction on stainless steel foams. In this contribution we present a study of the influence of the temperature of the activation (oxidation and reduction) stage on the type and morphology of the carbonaceous materials formed. The results obtained allow us to determine the optimal operating conditions to maximize the amount and the selectivity of the process to obtain a given type of CNM.
September, 2016 · DOI: 10.1016/j.cattod.2016.02.063
Química de Superficies y Catálisis
Impact of structured catalysts in amine oxidation under mild conditions
J.L. Santos; P. Navarro; J.A. Odriozola; M.A. Centeno; O.D. Pavel; B. Jurca; V.I. PàrvulescuCatalysis Today, 273 (2016) 266-272 DOI: 10.1016/j.cattod.2016.05.001
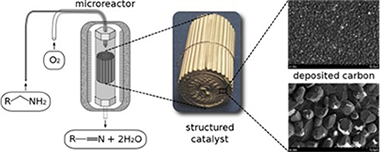
Abstract
A structured graphene/graphite catalyst grown on a commercial austenitic stainless steel sheet providing a micromonolith was obtained by submitting the nude stainless steel structure to a carbon-rich atmosphere (first 300 mL/min of a reductive H-2/N-2 (1:1) flow, then to 180 mL/min of a CH4/H-2 (1:5)) at high temperature (900 degrees C) for 2 h. The preparation procedure resulted in a homogenous surface coated with a carbon-rich film as observed by EDX and SEM images. Further characterizations by Raman spectroscopy revealed characteristic Raman lines of graphene and crystalline graphite disposed in a hierarchical organization. The disposal of the obtained surface layers was also confirmed by grazing incidence X-ray diffraction. Besides this, XRD indicated the overlapping diffraction lines of graphite, cementite and M7C3 carbides. The graphene nature of the outermost layer was also confirmed by XPS. The catalytic behavior of the structured graphene/graphite catalyst was evaluated in the selective oxidation of heptylamine. At 200 degrees C it afforded a total conversion with a combined selectivity in heptanonitrile and N-heptylidene-heptylamine of 67% (10% heptanonitrile) that corresponds indeed to a very efficient system in the absence of any metal. Kinetic experiments with the scope to calculate the activation energies were also performed.
September, 2016 · DOI: 10.1016/j.cattod.2016.05.001
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
Photocatalytic Escherichia coli inactivation by means of trivalent Er3+, Y3+ doping of BiVO4 system
Adan, C; Marugan, J; Obregon, S; Colon, GApplied Catalysis A-General, 526 (2015) 126-131 DOI: 10.1016/j.apcata.2016.08.002
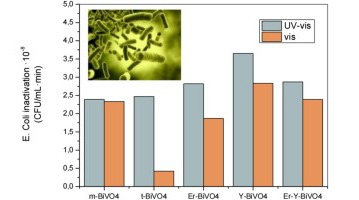
Abstract
BiVO4 samples doped with different contents of Er3+ and Y3+ were prepared by a simple surfactant free hydrothermal method. X-ray diffraction reveals that the doped materials consist of a heterogeneous structure formed by a mixture of tetragonal and monoclinic phases, being found Er3+ and Y3+ co-doping clearly stabilize the tetragonal structure of BiVO4. The monoclinic BiVO4 samples shows a strong absorption in the visible light region leading to band-gap values of around 2.4eV while the tetragonal BiVO4 displays higher band-gap values of 2.9 eV. The photocatalytic activity of the catalysts was investigated for the oxidation of methanol and inactivation of Escherichia coli showing that all the BiVO4 catalysts are photocatalytically active in the oxidation of methanol and are able to inactivate more than 99.99% of bacteria not only under UV light but also under visible light irradiation. The results revealed that the co-doping of Er3+ and Y3+ into BiVO4 exhibited enhanced photocatalytic activity for methanol oxidation under simulated solar light irradiation. The inactivation of E.coli show similar results for the doped systems although in relative terms of activity the Er3+,Y3+-BiVO4 sample show a better use of the visible light, leading to a higher activity than P25-TiO2.
September, 2016 · DOI: 10.1016/j.apcata.2016.08.002
Materiales de Diseño para la Energía y Medioambiente
Sliding wear resistance of porous biomorphic sic ceramics
Lopez-Robledo, MJ; Gomez-Martin, A; Ramirez-Rico, J; Martinez-Fernandez, JInternational Journal of Refractory Metals & Hard Materials, 59 (2016) 26-31 DOI: 10.1016/j.ijrmhm.2016.05.004
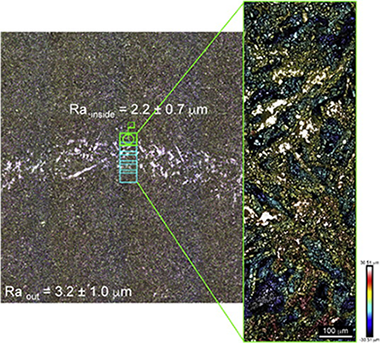
Abstract
Porous biomorphic SiC ceramics were fabricated from four different wood precursors, three natural woods and one recycled wood product, by reactive infiltration of molten silicon into a carbon preform obtained from wood pyrolysis. Sliding wear resistance when sliding against a Si3N4 ball in air was studied. Tribological experiments were done with a pin-on-disk apparatus, under normal loads of 1 and 2 N, at a sliding velocity of 100 mm/s. The wear properties and the volume fraction of porosity were correlated. A commercial sintered SiC ceramic was also tested for comparison. The measured values of friction coefficient were in the range reported in literature for monolithic SiC ceramics under similar dry contact conditions. Two concurrent wear mechanisms are taking place: abrasion from the SiC debris and soft ploughing. The presence of an oxide tribolayer was assessed using energy dispersive X-ray analysis. Wear rates were found to scale with the composite porosity.
September, 2016 · DOI: 10.1016/j.ijrmhm.2016.05.004
Nanotecnología en Superficies y Plasma
Effect of Nickel and Magnesium on the Electrochemical Behavior of AA 1050 Alloys in Nitric Acid Solution
Garcia-Garcia, FJ; Skeldon, P; Thompson, GEJournal of the Electrochemical Society, 163 (9) (2016) C593-C601 DOI: 10.1149/2.1181609jes
Abstract
The study investigates the influence of nickel and magnesium additions to AA 1050 aluminum alloy on the electrochemical behavior of the alloy in nitric acid solution under conditions relevant to the lithographic and electronic industries. Magnesium and nickel additions are of interest, since they can improve the alloy properties for the printing process by improving reverse bending fatigue strength and thermal softening resistance, while nickel may provide uniform pitting during electrograining. Scanning electron microscopy was used to characterize the resulting surface morphologies. The addition of nickel led to an increase in the pitting and corrosion potentials; additionally, it reduced the rate of dissolution of intermetallic particles during anodic polarization and increased the rate of aluminum dissolution during cathodic polarization. In contrast, the addition of magnesium had negligible influence on the open circuit and pitting behaviors, since the magnesium is retained in solid solution and has negligible influence on the cathodic behavior of intermetallic particles, which dominate the corrosion behavior.
September, 2016 · DOI: 10.1149/2.1181609jes
Materiales Coloidales
Microstructural, spectroscopic, and antibacterial properties of silver-based hybrid nanostructures biosynthesized using extracts of coriander leaves and seeds
Luna, C; Barriga-Castro, ED; Gomez-Trevino, A; Nuñez, NO; Mendoza-Resendez, RInternational Journal of Nanomedicine, 11 (2016) 4787-4798 DOI: 10.2147/IJN.S105166
Abstract
Coriander leaves and seeds have been highly appreciated since ancient times, not only due to their pleasant flavors but also due to their inhibitory activity on food degradation and their beneficial properties for health, both ascribed to their strong antioxidant activity. Recently, it has been shown that coriander leaf extracts can mediate the synthesis of metallic nanoparticles through oxidation/reduction reactions. In the present study, extracts of coriander leaves and seeds have been used as reaction media for the wet chemical synthesis of ultrafine silver nanoparticles and nanoparticle clusters, with urchin-and tree-like shapes, coated by biomolecules (mainly, proteins and polyphenols). In this greener route of nanostructure preparation, the active biocompounds of coriander simultaneously play the roles of reducing and stabilizing agents. The morphological and microstructural studies of the resulting biosynthesized silver nanostructures revealed that the nanostructures prepared with a small concentration of the precursor Ag salt (AgNO3 =5 mM) exhibit an ultrafine size and a narrow size distribution, whereas particles synthesized with high concentrations of the precursor Ag salt (AgNO3 =0.5 M) are polydisperse and formation of supramolecular structures occurs. Fourier transform infrared and Raman spectroscopy studies indicated that the bioreduction of the Ag- ions takes place through their interactions with free amines, carboxylate ions, and hydroxyl groups. As a consequence of such interactions, residues of proteins and polyphenols cap the biosynthesized Ag nanoparticles providing them a hybrid core/shell structure. In addition, these biosynthesized Ag nanomaterials exhibited size-dependent plasmon extinction bands and enhanced bactericidal activities against both Gram-positive and Gram-negative bacteria, displaying minimal inhibitory Ag concentrations lower than typical values reported in the literature for Ag nanoparticles, probably due to the synergy of the bactericidal activities of the Ag nanoparticle cores and their capping ligands.
September, 2016 · DOI: 10.2147/IJN.S105166
Reactividad de Sólidos
On the relevant role of solids residence time on their CO2 capture performance in the Calcium Looping technology
Perejon, A; Miranda-Pizarro, J; Perez-Maqueda, LA; Valverde, JMEnergy, 113 (2016) 160-171 DOI: 10.1016/j.energy.2016.07.028
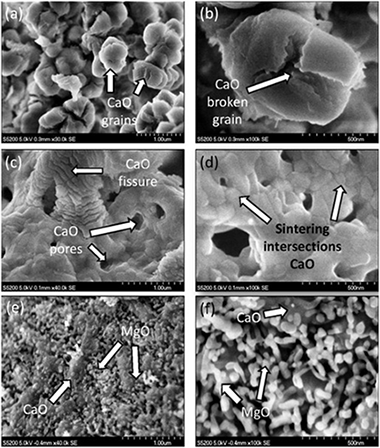
Abstract
The multicycle CO2 capture performance of CaO derived from natural limestone and dolomite has been investigated by means of thermogravimetry under realistic Calcium-Looping conditions, which necessarily involve high CO2 concentration and high temperatures in the calcination stage and fast transitions between the carbonation and calcination stages. Natural dolomite allows reducing the calcination temperature as compared to limestone while high calcination efficiency is maintained. This could help reducing the energy penalty of the CaL process thus further enhancing the industrial competitiveness for the integration of this technology into fossil fuel power plants. Importantly, the CO2 capture capacity of the sorbents is critically affected by the solids residence time in the carbonation and calcination stages within the feasible range in practice. Thus, carbonation/calcination residence times play a critical role on the multicycle CO2 capture performance, which has been generally dismissed in previous studies. A main observation is the enhancement of carbonation in the solid-state diffusion controlled phase, which is against the commonly accepted conception that the only relevant phase in the carbonation stage is the fast reaction-controlled stage on the surface of the solids. Thus, the CO2 capture efficiency may be significantly enhanced by increasing the solids residence time in the carbonator.
September, 2016 · DOI: 10.1016/j.energy.2016.07.028
Nanotecnología en Superficies y Plasma
Metallization of ceramic substrates by laser induced decomposition of coordination complexes
Rico, V; Lopez-Gascon, C; Espinos, JP; Lahoz, R; Laguna, M; Gonzalez-Elipe, AR; de la Fuente, GFJournal of the European Ceramic Society, 36 (2016) 2831-2836 DOI: 10.1016/j.jeurceramsoc.2016.04.016
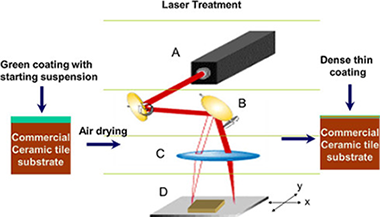
Abstract
This work describes an in-situ Nd:YAG laser-assisted coating method to modify industrial glazed ceramic surfaces. The method makes use of a Cu polymer coordination complex, transformed via 1064 nm continuos wave (cw) laser irradiation, into a lustre-type glassy coating covering the ceramic substrate. The obtained coatings, with typical thicknesses ranging between 4 and 14 μm, become integrated onto the ceramic glaze via a sharp interface, as found by SEM observation. Diffuse Reflectance UV-vis spectroscopy shows that the lustre effect arises from surface plasmon resonant effects associated to the formation of nanometric size Cu particles dispersed throughout the glaze coating. This was confirmed by XPS analysis and other techniques showing that the laser decomposition treatment induces the redox transformation of the Cu (II) complexes, present in the original precursor, into reduced Cu (0) nanoparticles.
September, 2016 · DOI: 10.1016/j.jeurceramsoc.2016.04.016
Nanotecnología en Superficies y Plasma
Stabilization of catalyst particles against sintering on oxide supports with high oxygen ion lability exemplified by Ir-catalyzed decomposition of N2O
Yentekakis, IV; Goula, G; Panagiotopoulou, P; Kampouri, S; Taylor, MJ; Kyriakou, G; Lambert, RMApplied Catalysis B-Environmental, 192 (2016) 357-364 DOI: 10.1016/j.apcatb.2016.04.011
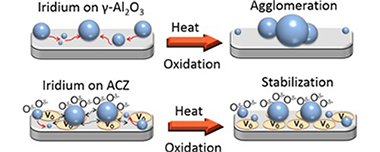
Abstract
Iridium nanoparticles deposited on a variety of surfaces exhibited thermal sintering characteristics that were very strongly correlated with the lability of lattice oxygen in the supporting oxide materials. Specifically, the higher the lability of oxygen ions in the support, the greater the resistance of the nanoparticles to sintering in an oxidative environment. Thus with gamma-Al2O3 as the support, rapid and extensive sintering occurred. In striking contrast, when supported on gadolinia-ceria and alumina-ceria-zirconia composite, the Ir nanoparticles underwent negligible sintering. In keeping with this trend, the behavior found with yttria-stabilized zirconia was an intermediate between the two extremes. This resistance, or lack of resistance, to sintering is considered in terms of oxygen spillover from support to nanoparticles and discussed with respect to the alternative mechanisms of Ostwald ripening versus nanoparticle diffusion. Activity towards the decomposition of N2O, a reaction that displays pronounced sensitivity to catalyst particle size (large particles more active than small particles), was used to confirm that catalytic behavior was consistent with the independently measured sintering characteristics. It was found that the nanoparticle active phase was Ir oxide, which is metallic, possibly present as a capping layer. Moreover, observed turnover frequencies indicated that catalyst-support interactions were important in the cases of the sinter-resistant systems, an effect that may itself be linked to the phenomena that gave rise to materials with a strong resistance to nanoparticle sintering.
September, 2016 · DOI: 10.1016/j.apcatb.2016.04.011
Nanotecnología en Superficies y Plasma
Reduced graphene oxide-carboxymethylcellulose layered with platinum nanoparticles/PAMAM dendrimer/magnetic nanoparticles hybrids. Application to the preparation of enzyme electrochemical biosensors
Borisova, B; Sanchez, A; Jimenez-Falcao, S; Martin, M; Salazar, P; Parrado, C; Pingarron, JM; Villalonga, RSensors and Actuators B-Chemical, 232 (2016) 84-90 DOI: 10.1016/j.snb.2016.02.106
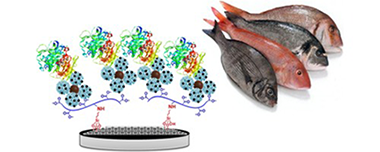
Abstract
The assembly of a novel layer-by-layer biosensor architecture using hybrid nanomaterials is explored for the construction of an amperometric enzyme biosensors. The nanostructured sensing interface was prepared with poly(dopamine)-modified magnetic nanoparticles which were covalently coated with four-generation ethylenediamine core polyamidoamine G-4 dendrimers and further decorated with platinum nanoparticles. This nanohybrid was fully characterized and further layered on glassy carbon electrodes coated with a graphene oxide-carboxymethylcellulose hybrid nanomaterial through electrostatic interactions. The nanostructured surface was then employed as scaffold for the covalent immobilization of the enzyme xanthine oxidase through a glutaraldehyde-mediated cross-linking. The enzyme electrode allowed the amperometric detection of xanthine in the 50 nM-12 mu M range, with a high sensitivity of 140 mA/M cm(2) and low detection limit of 13 nM. The biosensor exhibited high reproducibility and repeatability, and was successfully tested for the quantification of xanthine in fish samples.
September, 2016 · DOI: 10.1016/j.snb.2016.02.106
Nanotecnología en Superficies y Plasma
Isotope labelling to study molecular fragmentation during the dielectric barrier discharge wet reforming of methane
Montoro-Damas, AM; Gomez-Ramirez, A; Gonzalez-Elipe, AR; Cotrino, JJournal of Power Sources, 325 (2016) 501-505 DOI: 10.1016/j.jpowsour.2016.06.028
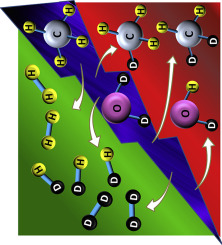
Abstract
Isotope labelling is used to study the wet plasma reforming of methane in a dielectric barrier discharge reactor using D2O and CH4 as reactants. Besides the formation of CO and hydrogen as main products, different partitions of H and D atoms are found in the hydrogen (i.e., Hz, HD, D-2), methane (i.e., CH4, CH3D and CH2D2) and water (D2O, DHO) molecules detected by mass spectrometry as outlet gases of the plasma process. The effect of operating parameters such as applied current, residence time and the addition of oxygen to the reaction mixture is correlated with the H/D distribution in these molecules, the overall reaction yield and the energetic efficiency of the process. The results prove the plasma formation of intermediate excited species that rendering water and methane instead of CO and hydrogen greatly contribute to decrease the overall energy efficiency of the reforming process.
September, 2016 · DOI: 10.1016/j.jpowsour.2016.06.028
Materiales Nanoestructurados y Microestructura
Timing of calcium nitrate addition affects morphology, dispersity and composition of bioactive glass nanoparticles
Zheng, K; Taccardi, N; Beltran, AM; Sui, BY; Zhou, T; Marthala, VRR; Hartmann, M; Boccaccini, ARRSC Advances, 6 (2016) 95101-95111 DOI: 10.1039/C6RA05548F
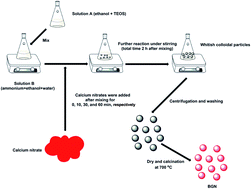
Abstract
Bioactive glass nanoparticles (BGN) are promising materials for a number of biomedical applications. Many parameters related to the synthesis of BGN using sol–gel methods can affect their characteristics. In this study, the influence of timing of calcium nitrate (calcium precursor) addition during processing on BGN characteristics was investigated. The results showed that the addition timing could affect the morphology, dispersity and composition of BGN. With delayed addition of calcium nitrate, larger, more regular and better dispersed BGN could be synthesized while the gap between nominal and actual compositions of BGN was widened. However, the addition timing had no significant influence on structural characteristics, as BGN with different addition-timing of calcium nitrate exhibited similar infrared spectra and amorphous nature. The results also suggested that monodispersed BGN could be synthesized by carefully controlling the addition of calcium nitrate. The synthesized monodispersed BGN could release Si and Ca ions continuously for up to at least 14 days. They also showed in vitro bioactivity and non-cytotoxicity towards rat bone marrow-derived mesenchymal stem cells (rBMSCs). In conclusion, the timing of calcium precursor addition is an essential parameter to be considered when producing BGN which should exhibit monodisperse characteristics for biomedical applications.
September, 2016 · DOI: 10.1039/C6RA05548F
Materiales de Diseño para la Energía y Medioambiente - Materiales Nanoestructurados y Microestructura
Monolithic supports based on biomorphic SiC for the catalytic combustion of hydrogen
Arzac, G. M.; Ramirez-Rico, J.; Gutierrez-Pardo, A.; Jimenez de Haro, M. C.; Hufschmidt, D.; Martinez-Fernandez, J.; Fernandez, A.RSC Advances, 6 (2016) 66373-66384 DOI: 10.1039/c6ra09127j
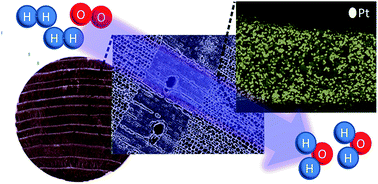
Abstract
Catalytic hydrogen combustion was studied with H-2/air mixtures in conditions that simulate the H-2 concentration of the exhaust gases from fuel cells (3-4% v/v H-2 in air). Pt-impregnated monoliths based on porous biomorphic SiC (bio-SiC) substrates were employed for the first time for this reaction. Capillary forces were exploited for the incipient impregnation of supports with H2PtCl6 solutions. Freeze drying permitted us to obtain a homogeneous distribution of the active phase reducing accumulation at the monolith's outer shell. The supports and catalysts were characterized from a structural and thermal point of view. Catalytic tests were performed in a homemade reactor fed with up to 1000 ml min(-1) H-2/air mixtures and a diffusional regime (non-isothermal) was achieved in the selected conditions. Catalyst loading was tested in the range of 0.25-1.5 wt% Pt and 100% conversion was achieved in all cases. Temperatures were recorded at different points of the monoliths during the reaction showing anisotropic thermal behavior for selected bio-SiC substrates. These effects are of interest for heat management applications and were explained in correlation with thermal conductivity measurements performed on the supports. Pt-impregnated monoliths were also tested in less than 100% conversion conditions (1% v/v H-2 in air) and in powder form in kinetic conditions for comparative purposes.
September, 2016 · DOI: 10.1039/c6ra09127j
Nanotecnología en Superficies y Plasma
Nanocolumnar association and domain formation in porous thin films grown by evaporation at oblique angles
Lopez-Santos, C; Alvarez, R; Garcia-Valenzuela, A; Rico, V; Loeffler, M; Gonzalez-Elipe, AR; Palmero, ANanotechnology, 27 (2016) 395702 DOI: 10.1088/0957-4484/27/39/395702
Abstract
Porous thin films grown at oblique angles by evaporation techniques are formed by tilted nanocolumnar structures which, depending on the material type and growth conditions, associate along certain preferential directions, giving rise to large domains. This arrangement, commonly denoted as bundling association, is investigated in the present work by performing fundamental experiments and growth simulations. It is proved that trapping processes of vapor species at the film surface, together with the shadowing mechanism, mediate the anisotropic widening of the nanocolumns and promote their preferential coalescence along certain directions, giving rise to domains with different shape and size. The role of these two processes is thoroughly studied in connection with the formation of these domains in materials as different as SiO2 and TiO2.
September, 2016 · DOI: 10.1088/0957-4484/27/39/395702
Nanotecnología en Superficies y Plasma
Laser Treatment of Nanoparticulated Metal Thin Films for Ceramic Tile Decoration
Rico, VJ; Lahoz, R; Rey-Garcia, F; Yubero, F; Espinos, JP; de la Fuente, GF; Gonzalez-Elipe, ARApplied Materials & Interfaces, 8 (2016) 24880-24886 DOI: 10.1021/acsami.6b07469
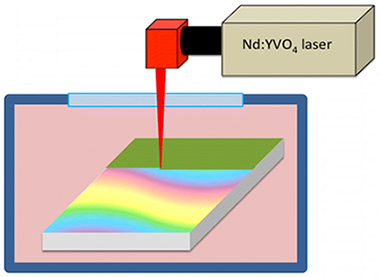
Abstract
This paper presents a new method for the fabrication of metal-like decorative layers on glazed ceramic tiles. It consists of the laser treatment of Cu thin films prepared by electron-beam evaporation at glancing angles. A thin film of discontinuous Cu nanoparticles was electron-beam-evaporated in an oblique angle configuration onto ceramic tiles and an ample palette of colors obtained by laser treatment both in air and in vacuum. Scanning electron microscopy along with UV–vis–near-IR spectroscopy and time-of-flight secondary ion mass spectrometry analysis were used to characterize the differently colored layers. On the basis of these analyses, color development has been accounted for by a simple model considering surface melting phenomena and different microstructural and chemical transformations of the outmost surface layers of the samples.
September, 2016 · DOI: 10.1021/acsami.6b07469
Reactividad de Sólidos
Assessment of the performance of commonly used DFT functionals vs. MP2 in the study of IL-Water, IL-Ethanol and IL-(H2O)(3) clusters
López-López, A., Ayala, R.Journal of Molecular Liquids, 220 (2016) DOI: 10.1016/j.molliq.2016.05.037
Abstract
We present a comparative study of the accuracy of different DFT approaches vs. MP2 for evaluating ionic liquids (ILs) + cosolvent. Namely, we are interested in [XBmim] + cosolvent (X being Cl-, BF4-, PF6-, and CH3SO3- anions and cosolvent being water or ethanol) and [XBmim] + (H2O)(3) clusters. In this study the B3LYP, B3LYP-D3, M06, M06-2X and M06-HF functionals with Pople and Dunning basis sets are considered. We find that the influence of the basis sets is a factor to take into consideration. As already seen for weakly bonded systems when the basis set quality is low the uncorrected counterpoise (unCP) or averaging counterpoise (averCP) energies must be used due to cancellation errors. Besides, the inclusion of extra diffuse functions and polarization is also required specially in the case of ILs interacting with water clusters. The B3LYP functional does not reproduce either the structure or the interaction energies for ILs + H2O and ILs + EtOH aggregates, the energetic discrepancies being more significant than the structural ones. Among the dispersive corrected functionals, M06-2X results resemble to a great extent the reference data when the unCP interaction energies are considered for both water and ethanol. In turn, M06 and B3LYP-D3 functionals are the best option for ILs containing polar and non-polar anions, respectively, whether the averCP interactions energies are taking into consideration. From the structural point of view, B3LYP and M06 functionals describe more open structures whereas B3LYP-D3, M06-2X and M06-HF structures resemble quite well MP2 results. When the number of water molecules increases the H bonding motif gains importance and the effect depends on the underlying functional. Only M06-2X and M06-HF behaviour is similar to that observed for one water molecule. This is important because to describe ILs-cosolvent solutions is not only necessary to take into account the ILs-cosolvent interactions but also the cosolvent-cosolvent ones in the ensemble of the system.
August, 2016 · DOI: 10.1016/j.molliq.2016.05.037
Effects of electronic and nuclear stopping power on disorder induced in GaN under swift heavy ion irradiation
Moisy, F; Sall, M; Grygiel, C; Balanzat, E; Boisserie, M; Lacroix, B; Simon, P; Monnet, INuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 381 (2016) 39-44 DOI: 10.1016/j.nimb.2016.05.024
Abstract
Wurtzite GaN epilayers, grown on the c-plane of sapphire substrate, have been irradiated with swift heavy ions at different energies and fluences, and thereafter studied by Raman scattering spectroscopy, UV–visible spectroscopy and transmission electron microscopy. Raman spectra show strong structural modifications in the GaN layer. Indeed, in addition to the broadening of the allowed modes, a large continuum and three new modes at approximately 200 cm−1, 300 cm−1 and 670 cm−1 appear after irradiation attributed to disorder-activated Raman scattering. In this case, spectra are driven by the phonon density of states of the material due to the loss of translation symmetry of the lattice induced by defects. It was shown qualitatively that both electronic excitations and elastic collisions play an important role in the disorder induced by irradiation. UV–visible spectra reveal an absorption band at 2.8 eV which is linked to the new mode at 300 cm−1observed in irradiated Raman spectra and comes from Ga-vacancies. These color centers are produced by elastic collisions (without any visible effect of electronic excitations).
August, 2016 · DOI: 10.1016/j.nimb.2016.05.024
Materiales Coloidales
Luminescent Rare-earth-based Nanoparticles: A Summarized Overview of their Synthesis, Functionalization, and Applications
Escudero, A; Carrillo-Carrion, C; Zyuzin, MV; Parak, WJTopics in current chemistry, 374 (2016) Article number 48 DOI: 10.1007/s41061-016-0049-8
Abstract
Rare-earth-based nanoparticles are currently attracting wide research interest in material science, physics, chemistry, medicine, and biology due to their optical properties, their stability, and novel applications. We present in this review a summarized overview of the general and recent developments in their synthesis and functionalization. Their luminescent properties are also discussed, including the latest advances in the enhancement of their emission luminescence. Some of their more relevant and novel biomedical, analytical, and optoelectronic applications are also commented on.
August, 2016 · DOI: 10.1007/s41061-016-0049-8
A new approach to the determination of the synthetic or natural origin of red pigments through spectroscopic analysis
Franquelo, ML; Perez-Rodriguez, JLSpectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 166 (2016) 103-111 DOI: 10.1016/j.saa.2016.04.054

Abstract
This work suggests a way of differentiation between the natural or synthetic origin of inorganic materials that were historically used in the Cultural Heritage field. An exhaustive review of different reported procedures of synthesis of pigments was conducted, as well as a review of the accompanying minerals in case of natural pigments. The natural or synthetic origin of the pigments studied in this work was performed through the characterization of the accompanying minerals, in the case of the natural pigments, or the trace elements that are present as part of synthesis by-products or washing/purifying reagents and/or reactants that have only been partly removed in the final steps of these processes. This work characterized red pigments due to their wide variety, complexity and possibility of use in different mixtures. The following pigments were studied: cinnabar-vermilion, red lead and iron pigments. Also mixtures of these pigments between them and with red lake were also studied. Natural cinnabar was accompanied by silicon oxide (opal, chalcedony or quartz), calcite, clay minerals and, sometimes, pyrite. K together with S indicated a synthetic pigment (vermilion) obtained through the wet method. Nevertheless, K has not been found in layers containing only vermilion in our samples. The presence of Sn in some cases indicated vermilion that came from the dry process. K from the synthesis always appeared in the red lead pigment. The red natural ochre was confirmed by presence of clay minerals and iron. It should be said that Ca and S, and sometimes Al and K, were usually found in Mars red pigment. The presence of Al and Ca allowed the identification of carmine lake.
August, 2016 · DOI: 10.1016/j.saa.2016.04.054
Materiales Avanzados
Correlation between chemical and mineralogical characteristics and permeability of phyllite clays using multivariate statistical analysis
Garzon, E; Romero, E; Sanchez-Soto, PJApplied Clay Science, 129 (2016) 92-101 DOI: 10.1016/j.clay.2016.05.008
Abstract
Phyllite clays are applied as a layer on a surface to be waterproofed and subsequently compacted. For this purpose, phyllite clays deposits can be grouped by their chemical and mineralogical characteristics, and these characteristics can be connected with their properties, mainly permeability, in order to select those deposits with the lowest permeability values. Several deposits of phyllite clays in the provinces of Almeria and Granada (SE Spain) have been studied. The results of applying a multivariate statistical analysis (MVA) to the chemical data analysed from 52 samples determined by XRF, mineralogical analysis by XRD and permeability are reported. Permeability, a characteristic physical property of phyllite clays, was calculated using the results for experimental nitrogen gas adsorption and nitrogen adsorption-desorption permeability dependence. According to the results, permeability values differentiated two groups, i.e. group 1 and group 2, with two subgroups in the latter. The influence of chemical as well as mineralogical characteristics on the permeability values of this set of phyllite clays was demonstrated using a multiple linear regression model. Two regression equations were deduced to describe the relationship between adsorption and desorption permeability values, which support this correlation. This was an indication of the statistical significance of each chemical and mineralogical variable, as it was added to the model. The statistical tests of the residuals suggested that there was no serious autocorrelation in the residuals.
August, 2016 · DOI: 10.1016/j.clay.2016.05.008
Materiales Ópticos Multifuncionales
Cellular Viscosity in Prokaryotes and Thermal Stability of Low Molecular Weight Biomolecules
Cuecas, A; Cruces, J; Galisteo-Lopez, JF; Peng, XJ; Gonzalez, JMBiophysical Journal, 111 (2016) 875–882 DOI: 10.1016/j.bpj.2016.07.024
Abstract
Some low molecular weight biomolecules, i.e., NAD(P)H, are unstable at high temperatures. The use of these biomolecules by thermophilic microorganisms has been scarcely analyzed. Herein, NADH stability has been studied at different temperatures and viscosities. NADH decay increased at increasing temperatures. At increasing viscosities, NADH decay rates decreased. Thus, maintaining relatively high cellular viscosity in cells could result in increased stability of low molecular weight biomolecules (i.e., NADH) at high temperatures, unlike what was previously deduced from studies in diluted water solutions. Cellular viscosity was determined using a fluorescent molecular rotor in various prokaryotes covering the range from 10 to 100°C. Some mesophiles showed the capability of changing cellular viscosity depending on growth temperature. Thermophiles and extreme thermophiles presented a relatively high cellular viscosity, suggesting this strategy as a reasonable mechanism to thrive under these high temperatures. Results substantiate the capability of thermophiles and extreme thermophiles (growth range 50–80°C) to stabilize and use generally considered unstable, universal low molecular weight biomolecules. In addition, this study represents a first report, to our knowledge, on cellular viscosity measurements in prokaryotes and it shows the dependency of prokaryotic cellular viscosity on species and growth temperature.
August, 2016 · DOI: 10.1016/j.bpj.2016.07.024
Materiales Ópticos Multifuncionales
Modified emission of extended light emitting layers by selective coupling to collective lattice resonances
Ramezani, Mohammad; Lozano, Gabriel; Verschuuren, Marc A.; Gomez-Rivas, JaimePhysical Review B, 94 (2016) 12 DOI: 10.1103/PhysRevB.94.125406
Abstract
We demonstrate that the coupling between light emitters in extended polymer layers and modes supported by arrays of plasmonic particles can be selectively enhanced by accurate positioning of the emitters in regions where the electric field intensity of a given mode is maximized. The enhancement, which we measure to reach up to 70%, is due to the improved spatial overlap and coupling between the optical mode and emitters. This improvement of the coupling leads to a modification of the emission spectrum and the luminous efficacy of the sample.
August, 2016 · DOI: 10.1103/PhysRevB.94.125406
Materiales de Diseño para la Energía y Medioambiente
Electrochemical Energy Storage Applications of CVD Grown Niobium Oxide Thin Films
Fiz, Raquel; Appel, Linus; Gutierrez-Pardo, Antonio; Ramirez-Rico, Joaquin; Mathur, SanjayACS Applied Materials & Interfaces, 8 (2016) 21423–21430 DOI: 10.1021/acsami.6b03945
Abstract
We report here on the controlled synthesis, characterization, and electrochemical properties of different polymorphs of niobium pentoxide grown by CVD of new single-source precursors. Nb2O5 films deposited at different temperatures showed systematic phase evolution from low-temperature tetragonal (TT-Nb2O5, T-Nb2O5) to high temperature monoclinic modifications (H–Nb2O5). Optimization of the precursor flux and substrate temperature enabled phase-selective growth of Nb2O5 nanorods and films on conductive mesoporous biomorphic carbon matrices (BioC). Nb2O5 thin films deposited on monolithic BioC scaffolds produced composite materials integrating the high surface area and conductivity of the carbonaceous matrix with the intrinsically high capacitance of nanostructured niobium oxide. Heterojunctions in Nb2O5/BioC composites were found to be beneficial in electrochemical capacitance. Electrochemical characterization of Nb2O5/BioC composites showed that small amounts of Nb2O5 (as low as 5%) in conjunction with BioCarbon resulted in a 7-fold increase in the electrode capacitance, from 15 to 104 F g–1, while imparting good cycling stability, making these materials ideally suited for electrochemical energy storage applications.
August, 2016 · DOI: 10.1021/acsami.6b03945
Reactividad de Sólidos
On the Multicycle Activity of Natural Limestone/Dolomite for Thermochemical Energy Storage of Concentrated Solar Power
Sarrion, B; Valverde, JM; Perejon, A; Perez-Maqueda, L; Sanchez-Jimenez, PEEnergy Technology, 4 (2016) 1013-1019 DOI: 10.1002/ente.201600068
Abstract
Cheap, efficient, and non-toxic energy storage technologies are urgently needed to handle the rapidly increasing penetration of intermittent renewable energies into the grid. This work explores the use of limestone and dolomite for energy storage in concentrated solar power (CSP) plants by means of the calcium looping (CaL) process based on the multicycle carbonation/calcination of CaO. An efficient integration of the CaL process into CSP plants involves high temperature carbonation and calcination at moderate temperatures in a close CO2 cycle for power generation. These conditions differ from those of the CaL process for CO2 capture, which lead to CaO deactivation as extensively reported in recent years. In contrast, we show that limestone- and dolomite-derived CaO give rise to a high residual conversion at CaL-CSP conditions and in short residence times, which would facilitate the development of a competitive and clean CSP technology with permanent energy storage.
August, 2016 · DOI: 10.1002/ente.201600068
Materiales Coloidales
Confinement and surface effects on the physical properties of rhombohedral-shape hematite (alpha-Fe2O3) nanocrystals
Luna, C; Cuan-Guerra, AD; Barriga-Castro, ED; Nunez, NO; Mendoza-Resendez, RMaterials Research Bulletin, 80 (2016) 44-52 DOI: 10.1016/j.materresbull.2016.03.029
Abstract
Morphological, microstructural and vibrational properties of hematite (alpha-Fe2O3) nanocrystals with a rhombohedral shape and rounded edges, obtained by forced hydrolysis of iron(III) solutions under a fast nucleation, have been investigated in detail as a function of aging time. These studies allowed us to propose a detailed formation mechanism and revealed that these nanocrystals are composed of four {104} side facets, two {110} faces at the edges of the long diagonal of the nanocrystals and two {-441} facets as the top and bottom faces. Also, the presence of nanoscopic pores and fissures was evidenced. The vibrational bands of such nanocrystals were shifted to lower frequencies in comparison with bulk hematite ones as the nanocrystal size was reduced due to phonon confinement effects. Also, the indirect and direct transition band gaps displayed interesting dependences on the aging time arising from quantum confinementand surface effects
August, 2016 · DOI: 10.1016/j.materresbull.2016.03.029
- ‹ previous
- 20 of 37
- next ›














