Artículos SCI
2019
2019
Nanotecnología en Superficies y Plasma
Graphene Formation Mechanism by the Electrochemical Promotion of a Ni Catalyst
Espinos, JP; Rico, VJ; Gonzalez-Cobos, J; Sanchez-Valencia, JR; Perez-Dieste, V; Escudero, C; de Lucas-Consuegra, A; Gonzalez-Elipe, ARACS Catalysis, 9 (2019) 11447-11454 DOI: 10.1021/acscatal.9b03820
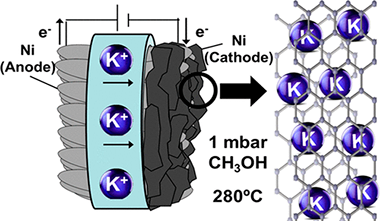
Abstract
In this work, we show that multilayer graphene forms by methanol decomposition at 280 degrees C on an electrochemically promoted nickel catalyst film supported on a K-beta Al2O3 solid electrolyte. In operando near ambient pressure photoemission spectroscopy and electrochemical measurements have shown that polarizing negatively the Ni electrode induces the electrochemical reduction and migration of potassium to the nickel surface. This elemental potassium promotes the catalytic decomposition of methanol into graphene and also stabilizes the graphene formed via diffusion and direct K-C interaction. Experiments reveal that adsorbed methoxy radicals are intermediate species in this process and that, once formed, multilayer graphene remains stable after electrochemical oxidation and back migration of potassium to the solid electrolyte upon positive polarization. The reversible diffusion of ca. 100 equivalent monolayers of potassium through the carbon layers and the unprecedented low-temperature formation of graphene and other carbon forms are mechanistic pathways of high potential impact for applications where mild synthesis and operation conditions are required.
Diciembre, 2019 · DOI: 10.1021/acscatal.9b03820
Materiales Coloidales
Monodisperse Gold Cuboctahedral Nanocrystals Directly Synthesized in Reverse Micelles: Preparation, Colloidal Dispersion in Organic Solvents and Water, Reversible Self-Assembly and Plasmonic Properties
Luna, C; Castaneda-Rodriguez, D; Barriga-Castro, ED; Nunez, NO; Mendoza-Resendez, RLangmuir, 34 (2019) 14291-14299 DOI: 10.1021/acs.langmuir.9b02374
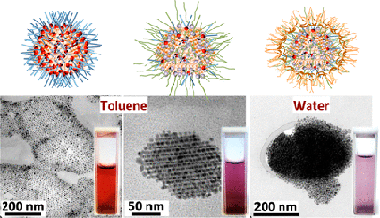
Abstract
The synthesis of organic-solvent-dispersible gold nanoparticles in reverse micelles of didodecyldimethylammonium bromide (DDAB) is revisited in the present investigation. Some parameters of synthesis, specifically the reaction volume and the concentration of the reducing agent, were slightly modified obtaining directly monodisperse gold nanocrystals (AuNCs) without the need to use additional active surfactants or additional treatments such as digestive ripening. Interestingly, most of the obtained AuNCs display the same exposed crystalline faces composed of six bounding facets (four {111} faces and two {002} faces), corresponding to single-crystalline face-centered cubic nanoparticles with a cuboctahedron shape. When these AuNCs are subsequently functionalized with 1-decanethiol (C10H21SH) or 1-dodecanethiol (C12H25SH), they don’t experience significant changes in their size or crystalline texture, however, they self-aggregate directly in the suspension at room temperature into faceted supramolecular structures and exhibit collective plasmonic excitations. Such self-organization is reversible under heating treatments allowing the observation of the influence of the AuNCs aggregation state on their plasmonic properties. Fourier transform infrared spectroscopy reveals that thiols only replace partially the DDAB molecules, and thus, DDAB molecules remain present in the thiol-capped AuNCs. To turn the thiol-capped nanocrystals into water-dispersible nanocrystals and extend their technological potential, they are stabilized with poloxamer 407 obtaining highly stable purple colloids in water.
Noviembre, 2019 · DOI: 10.1021/acs.langmuir.9b02374
Materiales Nanoestructurados y Microestructura
Morphological effects on the photocatalytic properties of SnO2 nanostructures
Kar, A; Olszowka, J; Sain, S; Sloman, SRI; Montes, O; Fernandez, A; Pradhan, SK; Wheatley, AEHJournal of Alloys and Compounds, 810 (2019) UNSP 151718 DOI: 10.1016/j.jallcom.2019.151718

Abstract
The photocatalytic properties of SnO2 nanocrystals are tuned by varying their morphology and microstructure. SnO2 nanoparticles and nanowedges have been synthesized using hydrothermal methods, while microwave irradiation techniques have given nanospheres. Detailed structural and chemical characterization of these different morphologies has been accomplished. The influence of SnO2 morphology on photocatalytic activity has been examined by monitoring the degradation of aqueous methylene blue dye. Results demonstrate that changing the morphology of the SnO2 modulates both surface area and levels of surface defects and that these alterations are reflected in the photocatalytic properties of the materials. The degradation of methylene blue dye (98%) in the presence of SnO2 nanoparticles under simulated solar irradiation is superior to previously reported photocatalyst performance and is comparable to that of standard TiO2 (Degussa P-25). The SnO2 nanoparticles perform better than both the nanowedges and nanospheres and this is attributed to the number of surface defects available to the high surface area material. They also reveal outstanding recyclability and stability.
Noviembre, 2019 · DOI: 10.1016/j.jallcom.2019.151718
Química de Superficies y Catálisis
Colombian metallurgical coke as catalysts support of the direct coal liquefaction
Rico, D; Agamez, Y; Romero, E; Centeno, MA; Odriozola, JA; Diaz, JDFuel, 255 (2019) 115748 DOI: 10.1016/j.fuel.2019.115748
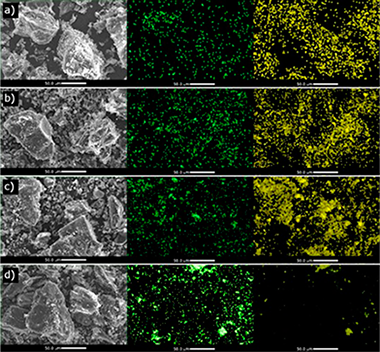
Abstract
A Colombian metallurgical coke was modified in its surface chemistry and was used as support of iron sulfide catalysts for direct coal liquefaction. The modification was made by treatments with diluted oxygen and HNO3 at different conditions. Changes in surface chemistry were studied by determining the point of zero charge (PZC), the isoelectric point (IEP), thermogravimetric analysis (TGA), temperature programmed decomposition-mass spectrometry (TPD-MS), Diffuse-reflectance infrared Fourier transform spectroscopy (DRIFTS) and nitrogen adsorption at 77 K. The results show that the materials obtained have a wide range of functional groups incorporated in a different proportion and quantity. The textural parameters indicate that treatment with diluted oxygen increases the surface area and incorporates micropores while the samples treated with HNO3 maintain the textural properties of the original material. The catalysts were also characterized by Raman spectroscopy. It was found that impregnation with the iron sulfide precursor does not significantly affect the Raman characteristics of the support. Additionally, XRD analysis shows smaller pyrite crystallites in the coke enriched with oxygenated groups of phenol and lactone indicating better dispersion of the active phase. The amount of oxygen chemisorbed per gram of catalyst shows that both, oxygen and nitric acid treatments, improve the relative dispersion of the active phase. It was found that the presence of the catalysts increases the conversion and yields towards oils and gases with respect to those of the tests without catalysts. Cokes modified by dilute oxygen gaseous treatment contain surface phenol and lactone groups and present the highest yield to oils.
Noviembre, 2019 · DOI: 10.1016/j.fuel.2019.115748
Nanotecnología en Superficies y Plasma
Ultrastable CoxSiyOz Nanowires by Glancing Angle Deposition with Magnetron Sputtering as Novel Electrocatalyst for Water Oxidation
Cano, M; Garcia-Garcia, FJ; Rodriguez-Padron, D; Gonzalez-Elipe, AR; Giner-Casares, JJ; Luque, RChemcatchem DOI: 10.1002/cctc.201901730

Abstract
Cobalt is one of the most promising non-noble metal as electrocatalyst for water oxidation. Herein, a highly stable silicon-cobalt mixed oxide thin film with a porous columnar nanostructure is proposed as electrocatalyst for oxygen evolution reaction (OER). CoOx and CoxSiyOz layers with similar thickness were fabricated at room temperature by magnetron sputtering in a glancing angle configuration (MS-GLAD) on tin-doped indium oxide (ITO) substrates. After characterization, a comparative study of the electrocatalytic performance for OER of both layers was carried out. The excellent long-term stability as electrocatalyst for OER of the porous CoxSiyOz thin film demonstrates that the presence of silicon on the mixed oxide network increases the mechanical stability of the Si/Co layer, whilst maintaining a considerable electrocatalytic response.
Noviembre, 2019 · DOI: 10.1002/cctc.201901730
Reactividad de Sólidos
Design of highly stabilized nanocomposite inks based on biodegradable polymer-matrix and gold nanoparticles for Inkjet Printing
Begines, Belen; Alcudia, Ana; Aguilera-Velazquez, Raul; Martinez, Guillermo; He, Yinfeng; Wildman, Ricky; Sayagues, Maria-Jesus; Jimenez-Ruiz, Aila; Prado-Gotor, RafaelScientific Reports, 9 (2019) 16097 DOI: 10.1038/s41598-019-52314-2
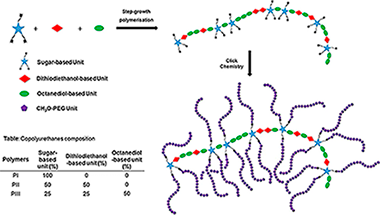
Abstract
Nowadays there is a worldwide growing interest in the Inkjet Printing technology owing to its potentially high levels of geometrical complexity, personalization and resolution. There is also social concern about usage, disposal and accumulation of plastic materials. In this work, it is shown that sugar-based biodegradable polyurethane polymers exhibit outstanding properties as polymer-matrix for gold nanoparticles composites. These materials could reach exceptional stabilization levels, and demonstrated potential as novel robust inks for Inkjet based Printing. Furthermore, a physical comparison among different polymers is discussed based on stability and printability experiments to search for the best ink candidate. The University of Seville logo was printed by employing those inks, and the presence of gold was confirmed by ToF-SIMS. This approach has the potential to open new routes and applications for fabrication of enhanced biomedical nanometallic-sensors using stabilized AuNP.
Noviembre, 2019 · DOI: 10.1038/s41598-019-52314-2
Materiales Avanzados
Phyllite clays as raw materials replacing cement in mortars: Properties of new impermeabilizing mortars
Arce, Carolina; Garzon, Eduardo; Sanchez-Soto, Pedro J.Construction and Building Materials, 224 (2019) 348-358 DOI: 10.1016/j.conbuildmat.2019.07.081

Abstract
The aim of this investigation was to determine the suitability of phyllite clays as a raw construction material. For that purpose, the cement in mortars was replaced by a phyllite clay (0–90 wt%) making this study the first of its kind to be performed. These materials were prepared with different water proportions according to the water content and water/cement and water/binder (cement plus phyllite clay) relationships. A comparative study of the most important properties of the resulting experimental mortars was carried out, such as apparent density, water retentivity, consistency and mechanical strength (flexural and compressive strength), along with an evaluation of the pozzolanic activity and permeability. The results showed that the increase of phyllite decreases the apparent density, the consistency and mechanical properties of the mortar, while water retentivity fluctuates. Good correlations (R2 > 0.84) were obtained between flexural and compressive strength for the mortars after 28 days of curing. Pozzolanic activity was observed at cement replacement of 80 wt% of phyllite. Moreover, new impermeabilizing mortars constituted by phyllite clay and cement have been obtained according to the low coefficients of permeability. Taking into account the findings of this research, phyllite clays can be applied as raw construction materials with savings derived from replacing cement in mortars and the low energy consumption involved in their production. However, the present study concluded that the use of phyllite clays did not improve the mechanical strength of these new mortars but, in contrast, they can be applied for impermeabilization purposes in Construction and Civil Engineering.
Noviembre, 2019 · DOI: 10.1016/j.conbuildmat.2019.07.081
Materiales Coloidales
Encapsulation of Upconversion Nanoparticles in Periodic Mesoporous Organosilicas
Rahmani, S; Jimenez, CM; Aggad, D; Gonzalez-Mancebo, D; Ocana, M; Ali, LMA; Nguyen, C; Nieto, AIB; Francolon, N; Oliveiro, E; Boyer, D; Mahiou, R; Raehm, L; Gary-Bobo, M; Durand, JO; Charnay, CMolecules, 24 (2019) 22 DOI: 10.3390/molecules24224054
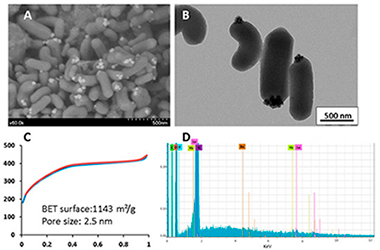
Abstract
(1) Background: Nanomedicine has recently emerged as a promising field, particularly for cancer theranostics. In this context, nanoparticles designed for imaging and therapeutic applications are of interest. We, therefore, studied the encapsulation of upconverting nanoparticles in mesoporous organosilica nanoparticles. Indeed, mesoporous organosilica nanoparticles have been shown to be very efficient for drug delivery, and upconverting nanoparticles are interesting for near-infrared and X-ray computed tomography imaging, depending on the matrix used. (2) Methods: Two different upconverting-based nanoparticles were synthesized with Yb3+-Er3+ as the upconverting system and NaYF4 or BaLuF5 as the matrix. The encapsulation of these nanoparticles was studied through the sol-gel procedure with bis(triethoxysilyl)ethylene and bis(triethoxysilyl)ethane in the presence of CTAB. (3) Results: with bis(triethoxysilyl)ethylene, BaLuF5: Yb3+-Er3+, nanoparticles were not encapsulated, but anchored on the surface of the obtained mesoporous nanorods BaLuF5: Yb3+-Er3+@Ethylene. With bis(triethoxysilyl)ethane, BaLuF5: Yb3+-Er3+ and NaYF4: Yb3+-Er(3+)nanoparticles were encapsulated in the mesoporous cubic structure leading to BaLuF5: Yb3+-Er3+@Ethane and NaYF4: Yb3+-Er3+@Ethane, respectively. (4) Conclusions: upconversion nanoparticles were located on the surface of mesoporous nanorods obtained by hydrolysis polycondensation of bis(triethoxysilyl)ethylene, whereas encapsulation occurred with bis(triethoxysilyl)ethane. The later nanoparticles NaYF4: Yb3+-Er3+@Ethane or BaLuF5: Yb3+-Er3+@Ethane were promising for applications with cancer cell imaging or X-ray-computed tomography respectively.
Noviembre, 2019 · DOI: 10.3390/molecules24224054
Reactividad de Sólidos
Graphene nanoplatelets for electrically conductive 3YTZP composites densified by pressureless sintering
Lopez-Pernia, C; Gallardo-Lopez, A; Morales-Rodriguez, A; Poyato, RJournal of the European Ceramic Society, 39 (2015) 4435-4439 DOI: 10.1016/j.jeurceramsoc.2019.05.067

Abstract
3 mol% yttria tetragonal zirconia polycrystalline (3YTZP) ceramic composites with 2.5, 5 and 10 vol% graphene nanoplatelets (GNP) were pressureless sintered in argon atmosphere between 1350 and 1450 degrees C. The effects of the GNP content and the sintering temperature on the densification, microstructure and electrical properties of the composites were investigated. An isotropic distribution of GNP surrounding ceramic regions was exhibited regardless the GNP content and sintering temperature used. Electrical conductivity values comparable to the ones of fully dense composites prepared by more complex techniques were obtained, even though full densification was not achieved. While the composite with 5 vol% GNP exhibited electrical anisotropy with a semiconductor-type behaviour, the composite with 10 vol% GNP showed an electrically isotropic metallic-type behaviour.
Noviembre, 2019 · DOI: 10.1016/j.jeurceramsoc.2019.05.067
Nanotecnología en Superficies y Plasma
Kinetic energy-induced growth regimes of nanocolumnar Ti thin films deposited by evaporation and magnetron sputtering
Alvarez, R.; Garcia-Valenzuela, A.; Rico, V; Garcia-Martin, J. M.; Cotrino, J.; Gonzalez-Elipe, A. R.; Palmero, A.Nanotechnology, 30 (2019) 475603 DOI: 10.1088/1361-6528/ab3cb2
Abstract
We experimentally analyze different growth regimes of Ti thin films associated to the existence of kinetic energy-induced relaxation mechanisms in the material's network when operating at oblique geometries. For this purpose, we have deposited different films by evaporation and magnetron sputtering under similar geometrical arrangements and at low temperatures. With the help of a well-established growth model we have found three different growth regimes: (i) low energy deposition, exemplified by the evaporation technique, carried out by species with typical energies in the thermal range, where the morphology and density of the film can be explained by solely considering surface shadowing processes, (ii) magnetron sputtering under weak plasma conditions, where the film growth is mediated by surface shadowing mechanisms and kinetic-energy-induced relaxation processes, and (iii) magnetron sputtering under intense plasma conditions, where the film growth is highly influenced by the plasma, and whose morphology is defined by nanocolumns with similar tilt than evaporated films, but with much higher density. The existence of these three regimes explains the variety of morphologies of nanocolumnar Ti thin films grown at oblique angles under similar conditions in the literature.
Noviembre, 2019 · DOI: 10.1088/1361-6528/ab3cb2
Materiales Ópticos Multifuncionales
Casimir-Lifshitz Force Based Optical Resonators
Esteso, V; Carretero-Palacios, S; Miguez, HJournal of Physical Chemistry Letters, 10 (2019) 5856-5860 DOI: 10.1021/acs.jpclett.9b02030
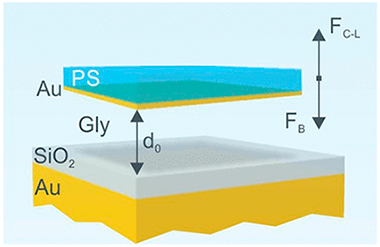
Abstract
We theoretically investigate the building of optical resonators based on the levitation properties of thin films subjected to strong repulsive Casimir-Lifshitz forces when immersed in an adequate medium and confronted with a planar substrate. We propose a design in which cavities supporting high Q-factor optical modes at visible frequencies can be achieved by means of combining commonly found materials, such as silicon oxide, polystyrene or gold, with glycerol as a mediating medium. We use the balance between flotation and repulsive Casimir-Lifshitz forces in the system to accurately tune the optical cavity thickness and hence its modes. The effects of other forces, such as electrostatic, that may come into play are also considered. Our results constitute a proof of concept that may open the route to the design of photonic architectures in environments in which dispersion forces play a substantial role and could be of particular relevance for devising novel microfluidic optical resonators.
Octubre, 2019 · DOI: 10.1021/acs.jpclett.9b02030
Reactividad de Sólidos
A QTAIM and DFT study of the dizinc bond in non-symmetric [CpZn2Ln] complexes
Ayala, R; Galindo, AJournal of Organometallic Chemistry, 898 (2019) UNSP 120878 DOI: 10.1016/j.jorganchem.2019.120878
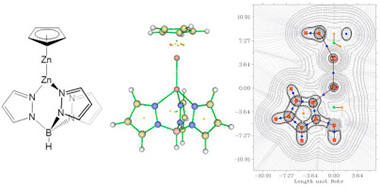
Abstract
Several [Zn2L2] and [CpZn2Ln] dizinc compounds have been studied by density functional theory (DFT) and quantum theory of atoms in molecules (QTAIM) in order to compare the nature and topology of the Zn-Zn bond in symmetrical and non-symmetrical complexes. The stability of these complexes have been evaluated on the basis of the formation energies. The disproportionation reaction has also been analysed indicating that symmetric complexes are less stable than non-symmetric ones. To certain extent, the properties of the [CpZn2Ln] complexes are between those of the [Zn2L2] and [Zn2Cp2] compounds. The asymmetry of the [CpZn2Ln] compounds is illustrated in terms of the topological properties, especially in the Source Function (SF) and Natural Bond Orbital (NBO) analysis.
Octubre, 2019 · DOI: 10.1016/j.jorganchem.2019.120878
Reactividad de Sólidos
Influence of pre-deformation on the precipitation hardening in Cu-Ni-Si alloy
Donoso, E; Dianez, MJ; Criado, JMRevista de Metalurgia, 55 (2019) e157 DOI: 10.3989/revmetalm.157
Abstract
The effects of pre-deformation on the precipitation processes in a Cu-2.8 Ni-1.4 Si (at.%) alloy were studied using differential scanning calorimetric (DSC), transmission electron microscopy (TEM) and microhardness measurements. The calorimetric curves shows the presence of one exothermic reaction attributed to the formation of delta-Ni2Si precipitates in the copper matrix that was confirmed by TEM. In addition it can be observed that the temperature of the maximum of the DSC peak decreases with the increase of the pre-deformation to the aging treatments. The activation energies calculated for the precipitation of by the Kissinger method, were similar to those calculated by an Arrhenius function, from the maximum hardening of the matrix due to aging treatments (saturation of the hardness during isothermal aging). The analysis of the microhardness measurements together with the calorimetric curves and the TEM micrographs confirm, on the one hand, that the formation of the delta-Ni2Si phase, during the aging treatments, are responsible for the hardening of the copper matrix, and on the other hand that the deformation prior to the aging treatment partially inhibits the formation of the precipitates.
Octubre, 2019 · DOI: 10.3989/revmetalm.157
Materiales Ópticos Multifuncionales
Spatially Resolved Analysis of Defect Annihilation and Recovery Dynamics in Metal Halide Perovskite Single Crystals
Galisteo-Lopez, JF; Calvo, ME; Miguez, HACS Applied Energy Materials, 2 (2019) 6967-6972 DOI: 10.1021/acsaem.9b01335
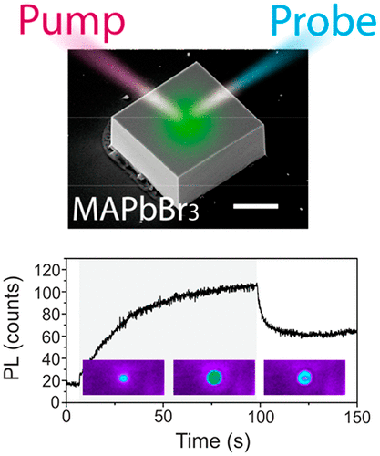
Abstract
The spectacular advances in efficiency of optoelectronic devices based on lead-halide perovskites have been accompanied by detailed structural and optical studies regarding the instability presented by these materials, which constitute their main bottleneck for commercialization. Following a pump and probe scheme in a laser scanning confocal microscope, we resolve the photoinduced emission activation/deactivation dynamics in CH3NH3PbBr3 single crystals with millisecond and sub-micrometer resolution. This is complemented with a study of spectral variations and interpreted in the framework of light-induced ion migration and associated defect passivation. Our results point to the presence of photoinduced structural changes accompanying the migration of ions.
Octubre, 2019 · DOI: 10.1021/acsaem.9b01335
Nanotecnología en Superficies y Plasma
Sodium ion storage performance of magnetron sputtered WO3 thin films
Garcia-Garcia, FJ; Mosa, J; Gonzalez-Elipe, AR; Aparicio, MElectrochimica Acta, 321 (2019) 134669 DOI: 10.1016/j.electacta.2019.134669
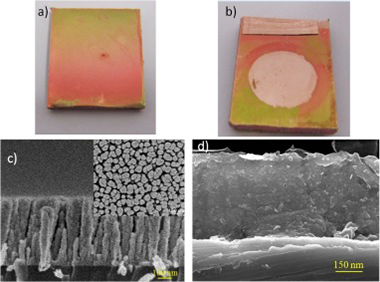
Abstract
WO3 thin film electrodes were successfully prepared by magnetron sputtering (MS) deposition under an oblique angle configuration (OAD). Intercalation of Na ions in the tungsten oxide layers has been studied using electrochemical techniques. Sample characterization before and after sodium intercalation has been carried out by Raman, XPS and XRD measurements. ToF-SIMS analysis has been also performed in order to analyze the element depth profiles along the electrode thickness. Electron microscopy evaluation of the cross section confirms the porous structure of the coatings. Batteries integrating these WO3 electrodes have a discharge capacity of 120 mA h g(-1) at the initial cycles and show an adequate capacity retention upon 300 cycles. The WO3-OAD thin-films are proposed as promising electrodes for Na-ion batteries.
Octubre, 2019 · DOI: 10.1016/j.electacta.2019.134669
Materiales Coloidales - Materiales Ópticos Multifuncionales
Synthesis, functionalization and properties of uniform europium-doped sodium lanthanum tungstate and molybdate (NaLa(XO4)(2), X = Mo,W) probes for luminescent and X-ray computed tomography bioimaging
Laguna, M; Nunez, NO; Becerro, AI; Lozano, G; Moros, M; de la Fuente, JM; Corral, A; Balcerzyk, M; Ocana, MJournal of Colloid and Interface Science, 554 (2019) 520-530 DOI: 10.1016/j.jcis.2019.07.031
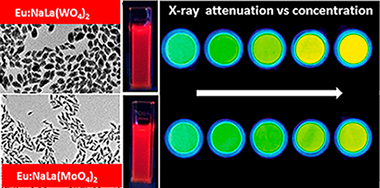
Abstract
A one-pot simple procedure for the synthesis of uniform, ellipsoidal Eu3+-doped sodium lanthanum tungstate and molybdate (NaLa(XO4)(2), X = W, Mo) nanophosphors, functionalized with carboxylate groups, is described. The method is based on a homogeneous precipitation process at 120 degrees C from appropriate Na+ Ln(3+) and tungstate or molybdate precursors dissolved in ethylene glycol/water mixtures containing poly acrylic acid. A comparative study of the luminescent properties of both luminescent materials as a function of the Eu3+ doping level has been performed to find the optimum nanophosphor, whose efficiency as X-ray computed tomography contrast agent is also evaluated and compared with that of a commercial probe. Finally, the cell viability and colloidal stability in physiological pH medium of the optimum samples have also been studied to assess their suitability for biomedical applications.
Octubre, 2019 · DOI: 10.1016/j.jcis.2019.07.031
Química de Superficies y Catálisis
The Success Story of Gold-Based Catalysts for Gas- and Liquid-Phase Reactions: A Brief Perspective and Beyond
Price, CAH; Pastor-Perez, L; Ivanova, S; Reina, TR; Liu, JFrontiers in Chemistry, 7 (2019) 691 DOI: 10.3389/fchem.2019.00691

Abstract
Gold has long held the fascination of mankind. For millennia it has found use in art, cosmetic metallurgy and architecture; this element is seen as the ultimate statement of prosperity and beauty. This myriad of uses is made possible by the characteristic inertness of bulk gold; allowing it to appear long lasting and above the tarnishing experienced by other metals, in part providing its status as the most noble metal.
Octubre, 2019 · DOI: 10.3389/fchem.2019.00691
Reactividad de Sólidos
The influence of mechanical activation process on the microstructure and mechanical properties of bulk Ti2AlN MAX phase obtained by reactive hot pressing
Salvo, C; Chicardi, E; Garcia-Garrido, C; Jimenez, JA; Aguilar, C; Usuba, J; Mangalaraja, RVCeramics International, 45 (2019) 17793-17799 DOI: 10.1016/j.ceramint.2019.05.350
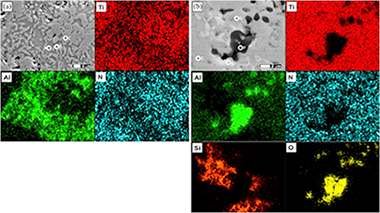
Abstract
The effect of mechanical activation process on the microstructure and mechanical properties of bulk nanostructured Ti2AlN compound has been investigated in this work. The mixture of Ti and AlN powders was prepared in a 2:1 molar ratio, and a part of this powder was subjected to a high-energy milling process under argon atmosphere for 10 h using agate as grinding media. Finally, the densification and formation of the ternary Ti2AlN MAX phase through solid state reaction of both unmilled and milled powders were carried out by hot pressing under 15 or 30 MPa at 1200 degrees C for 2 h. The microstructure of precursor powder mixtures and the consolidated samples was characterized by using X-ray diffraction (XRD) and a scanning electron microscope equipped with an energy dispersive X-ray spectroscopy (SEM/EDS). The X-ray diffraction patterns were fitted using the Rietveld refinement for phase quantification and to determine their most important microstructural parameters. Microstructure and mechanical properties of the consolidated samples were correlated with the load used for the hot pressing process. The substantial increase of hardness, the higher densification and the lower grain sizes observed in the samples prepared from the activated powders were attributed to the formation of second phases like Ti5Si3 and Al2O3.
Octubre, 2019 · DOI: 10.1016/j.ceramint.2019.05.350
Propiedades mecánicas, modelización y caracterización de cerámicos avanzados
Sintering kinetics, defect chemistry and room-temperature mechanical properties of titanium nitride prepared by spark plasma sintering
Chavez, JMM; Moshtaghioun, BM; Hernandez, FLC; Garcia, DGJournal of Alloys and Compounds, 807 (2019) 151666 DOI: 10.1016/j.jallcom.2019.151666
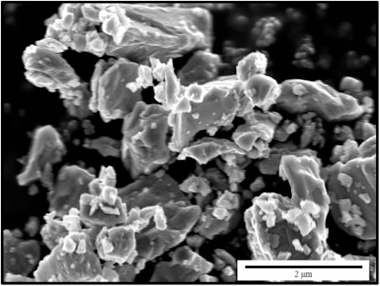
Abstract
Fully dense titanium nitride polycrystals have been prepared by spark plasma sintering. The kinetics of the sintering process and the optimized conditions for SPS processing have been put forward. Microstructural analyses of the resulting samples have unambiguously shown the coexistence of titanium as Ti2+, Ti3+ and Ti4+, thus driving the presence of cation vacancies. This fact is a new ingredient which is shown to influence the mechanical properties of this strategic ceramic.
Octubre, 2019 · DOI: 10.1016/j.jallcom.2019.151666
Reactividad de Sólidos
The Calcium-Looping (CaCO3/CaO) process for thermochemical energy storage in Concentrating Solar Power plants
Ortiz, C; Valverde, JM; Chacartegui, R; Perez-Maqueda, LA; Gimenez, PRenewable & Sustanaible Energy Reviews, 113 (2019) 109252 DOI: 10.1016/j.rser.2019.109252
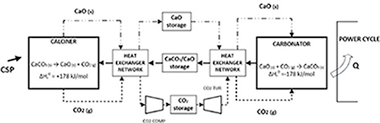
Abstract
Energy storage based on thermochemical systems is gaining momentum as a potential alternative to molten salts in Concentrating Solar Power (CSP) plants. This work is a detailed review about the promising integration of a CaCO3/CaO based system, the so-called Calcium-Looping (CaL) process, in CSP plants with tower technology. The CaL process relies on low cost, widely available and non-toxic natural materials (such as limestone or dolomite), which are necessary conditions for the commercial expansion of any energy storage technology at large scale. A comprehensive analysis of the advantages and challenges to be faced for the process to reach a commercial scale is carried out. The review includes a deep overview of reaction mechanisms and process integration schemes proposed in the recent literature. Enhancing the multicycle CaO conversion is a major challenge of the CaL process. Many lab-scale analyses carried out show that residual effective CaO conversion is highly dependent on the process conditions and the CaO precursors used, reaching values in a wide range (0.07–0.82). The selection of the optimal operating conditions must be based on materials performance, process integration, technology and economics aspects. Global plant efficiencies over 45% (without considering solar-side losses) show the interest of the technology. Furthermore, the technological maturity and potential of the process is assessed. The direction towards which future works should be headed is discussed.
Octubre, 2019 · DOI: 10.1016/j.rser.2019.109252
Nanotecnología en Superficies y Plasma
Influence of Titanium Oxide Pillar Array Nanometric Structures and Ultraviolet Irradiation on the Properties of the Surface of Dental Implants-A Pilot Study
Leon-Ramos, JR; Diosdado-Cano, JM; Lopez-Santos, C; Barranco, A; Torres-Lagares, D; Serrera-Figallo, MANanomaterials, 9 (2019) 1458 DOI: 10.3390/nano9101458

Abstract
Aim: Titanium implants are commonly used as replacement therapy for lost teeth and much current research is focusing on the improvement of the chemical and physical properties of their surfaces in order to improve the osseointegration process. TiO2, when it is deposited in the form of pillar array nanometric structures, has photocatalytic properties and wet surface control, which, together with UV irradiation, provide it with superhydrophilic surfaces, which may be of interest for improving cell adhesion on the peri-implant surface. In this article, we address the influence of this type of surface treatment on type IV and type V titanium discs on their surface energy and cell growth on them. Materials and methods: Samples from titanium rods used for making dental implants were used. There were two types of samples: grade IV and grade V. In turn, within each grade, two types of samples were differentiated: untreated and treated with sand blasting and subjected to double acid etching. Synthesis of the film consisting of titanium oxide pillar array structures was carried out using plasma-enhanced chemical vapor deposition equipment. The plasma was generated in a quartz vessel by an external SLAN-1 microwave source with a frequency of 2.45 GHz. Five specimens from each group were used (40 discs in total). On the surfaces to be studied, the following determinations were carried out: (a) X-ray photoelectron spectroscopy, (b) scanning electron microscopy, (c) energy dispersive X-ray spectroscopy, (d) profilometry, (e) contact angle measurement or surface wettability, (f) progression of contact angle on applying ultraviolet irradiation, and (g) a biocompatibility test and cytotoxicity with cell cultures. Results: The application of ultraviolet light decreased the hydrophobicity of all the surfaces studied, although it did so to a greater extent on the surfaces with the studied modification applied, this being more evident in samples manufactured in grade V titanium. In samples made in grade IV titanium, this difference was less evident, and even in the sample manufactured with grade IV and SLA treatment, the application of the nanometric modification of the surface made the surface optically less active. Regarding cell growth, all the surfaces studied, grouped in relation to the presence or not of the nanometric treatment, showed similar growth. Conclusions. Treatment of titanium oxide surfaces with ultraviolet irradiation made them change temporarily into superhydrophilic ones, which confirms that their biocompatibility could be improved in this way, or at least be maintained.
Octubre, 2019 · DOI: 10.3390/nano9101458
Nanotecnología en Superficies y Plasma
Highly selective few-ppm NO gas-sensing based on necklace-like nanofibers of ZnO/CdO n-n type I heterojunction
Naderi, H; Hajati, S; Ghaedi, M; Espinos, JPSensors and Actuators B-Chemical, 297 (2019) 126774 DOI: 10.1016/j.snb.2019.126774
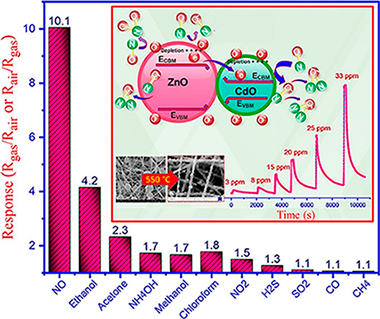
Abstract
Electrospinning method followed by calcination is applied to synthesize ZnO/CdO nanofibers. Characterization is performed by X-ray diffraction (XRD), scanning electron microscopy (SEM), field emission scanning electron microscopy (FESEM), energy dispersive spectroscopy (EDS), diffuse reflectance spectroscopy (DRS), X-ray photoelectron spectroscopy (XPS), ultraviolet photoelectron spectroscopy (UPS) and reflection electron energy loss spectroscopy (REELS), which resulted in detailed analysis of the sensing material. For instance, it was found that the ZnO/CdO is n-n type I heterojunction which possesses straddling energy band gap, which could affect the mechanism of gas sensing. An electroless gold-plated interdigitated electrode with spacing 200 mu m is fabricated on alumina substrate to host the designed nanofibers being used as gas sensor. Gas-sensing activity of the heterojunction is investigated against NO, NO2, H2S, CH4, SO2 and CO in addition to VOCs such as ethanol, acetone, ammonia, methanol, and chloroform with high selectivity and response to NO gas by monitoring resistance changes. Detailed discussion on the mechanism of sensing is presented. The ZnO/CdO nanofibers are found to be highly sensitive to very low concentration range of NO gas (1.2-33 ppm) at optimal operating temperature of 215 degrees C. The influence of humidity (20-96%) on the sensor response was found to be ignorable. Additionally, good repeatability and long-term stability (45 days, every 5 days, SD = 0.7) was obtained for this sensor. Typically, short response times of 47 and 35 s are obtained versus 3 and 33 ppm of NO, respectively, making our sensor promisingly applicable for monitoring this toxic gas in polluting industries, metropolises and maybe in exhaled breath.
Octubre, 2019 · DOI: 10.1016/j.snb.2019.126774
Química de Superficies y Catálisis
Effect of starch as binder in carbon aerogel and carbon xerogel preparation
Rodriguez, N; Agamez-Pertuz, YY; Romero, E; Diaz-Velasquez, JD; Odriozola, JA; Centeno, MAJournal of Non-Crystalline Solids, 522 (2019) UNSP 119554 DOI: 10.1016/j.jnoncrysol.2019.119554

Abstract
Carbon aerogels and carbon xerogels were synthesized through resorcinol - formaldehyde polycondensation using Na2CO3 as catalyst. The effect of soluble starch introduction in the organic gel preparation on the porous surface properties of these materials was studied. The role of the drying process of the organic gels on the changes in the surface and structural properties of these materials after the addition of soluble starch is discussed. The presence of starch in the prepared carbon xerogels results in the development of microporosity while maintaining the characteristic mesoporosity of carbon xerogels. The Brunauer - Emmett -Teller (BET) surface area increases from 309 m(2)/g in carbon xerogel without soluble starch until 685 m(2)/g when 10% of soluble starch is added. The R- value and average crystallite lattice parameters, inter-layer spacing, crystallite height, crystallite diameter and the average number of aromatic layers per carbon crystallite are discussed in function of drying step and presence of soluble starch. The surface properties were also studied by Raman and DRIFT spectroscopies.
Octubre, 2019 · DOI: 10.1016/j.jnoncrysol.2019.119554
Fotocatálisis Heterogénea: Aplicaciones
Comparison of the effects generated by the dry-soft grinding and the photodeposition of Au and Pt processes on the visible light absorption and photoactivity of TiO2
Galeano, L; Valencia, S; Marin, JM; Restrepo, G; Navio, JA; Hidalgo, MCMaterials Research Express, 6 (2019) 1050d9 DOI: 10.1088/2053-1591/ab4316
Abstract
The influence of dry-soft grinding and photodeposition of gold (Au) or platinum (Pt) in the improvement of the photoactivity of TiO2 synthesized by an integrated sol-gel and solvothermal method was studied. TiO2 was modified by a dry-soft grinding process in a planetary ball mill (TiO2(G)). Subsequently, Au or Pt particles were photodeposited in both unmodified TiO2 and TiO2(G) obtaining Au-TiO2, Pt-TiO2, Au-TiO2(G), and Pt-TiO2(G) materials. The photoactivity of the materials was evaluated in the phenol photodegradation under simulated solar radiation. Pt-TiO2 showed the greatest degree of photoactivity improvement in comparison with TiO2 and TiO2-P25. The dry-soft grinding process led to a high photocatalytic activity of TiO2(G) that was similar to Pt-TiO2 activity as consequence of a slight increase in the crystallinity in TiO2(G) due to an additional anatase formation in comparison with TiO2. However, further photocatalytic improvement in TiO2(G) were not achieved with the addition of Au or Pt. Therefore, the dry-soft grinding treatment and noble metal deposition led to similar improvements in the photocatalytic activity of TiO2 for phenol oxidation.
Octubre, 2019 · DOI: 10.1088/2053-1591/ab4316
Materiales Coloidales
From structure to luminescence investigation of oxyfluoride transparent glasses and glass-ceramics doped with Eu3+/Dy3+ ions
Walas, M; Lisowska, M; Lewandowski, T; Becerro, AI; Lapinski, M; Synak, A; Sadowski, W; Koscielska, BJournal of Alloys and Compounds, 896 (2019) 1410-1418 DOI: 10.1016/j.jallcom.2019.07.017
Abstract
Glasses and glass-ceramics with nominal composition 73 TeO2- 4BaO-3Bi(2)O(3)-18SrF(2)-2RE(2)O(3) (where RE = Eu, Dy) have been synthesized by conventional melt-quenching technique and subsequent heat treatment at 370 degrees C for 24 h in air atmosphere. Various Eu3+ to Dy3+ molar ratio have been applied to investigate luminescence properties in both glass and glass-ceramic matrices. Especially, white light emission through simultaneous excitation of Eu3+ and Dy3+ has been studied in detail. Influence of crystalline SrF2 phase on luminescence kinetics has been determined by luminescence decay time measurements. Presence of crystalline SrF2 phase has been confirmed by X-ray diffraction technique XRD and transmission electron microscopy TEM. X-ray photoelectron spectroscopy XPS and Fourier-transform infrared spectroscopy FTIR have been applied to get further insight into structural properties of glass and glass-ceramic materials. Color tunable white light emission has been obtained using UV excitation. Influence of the SrF2 crystallization on luminescence properties of prepared materials have been described in detail. Moreover, luminescence properties and especially emission color dependence on the Eu3+ to Dy3+ molar ratio have been exhaustively studied. Color-tunable white light emission has been observed as a result of simultaneous radiative transition of both, Eu3+ and Dy3+ ions when applying UV excitation. Judd - Ofelt and other optical parameters have been calculated based on luminescence emission spectra. Achieved results confirm that tellurite glass-ceramics containing SrF2 nanocrystals are good hosts for RE3+ ions and they can be considered as new phosphors for white light emitting diodes WLEDs.
Octubre, 2019 · DOI: 10.1016/j.jallcom.2019.07.017
Materiales de Diseño para la Energía y Medioambiente
Applications and potentialities of Atomic Force Microscopy in fossil and extant plant cuticle characterization
Benitez, JJ; Guzman-Puyol, S; Dominguez, E; Heredia, A; Heredia-Guerrero, JAReview of Palaeobotany and Palynology, 268 (2019) 125-132 DOI: 10.1016/j.revpalbo.2019.06.015
Abstract
Atomic Force Microscopy (AFM) is a versatile technique of surface characterization, providing accurate information about the topography and other wide variety of magnitudes at submicron scale. It is extensively utilized in materials science, but its use in other disciplines such as paleobotany is infrequent. In this review, we introduce the main concepts of AFM to paleobotanists, comparing the characteristics of this technique to common electronic and optical microscopies. Then, main works with extant plants, in particular plant cuticles, are described. Finally, realistic applications with fossils are reviewed and their potential use in the characterization of plant fossils discussed. AFM is proposed as a complementary technique to common microscopies to characterize plant cuticle fine details at nanoscale.
Septiembre, 2019 · DOI: 10.1016/j.revpalbo.2019.06.015
Nanotecnología en Superficies y Plasma
SiOx by magnetron sputtered revisited: Tailoring the photonic properties of multilayers
Garcia-Valenzuela, A; Alvarez, R; Espinos, JP; Rico, V; Gil-Rostra, J; Palmero, A; Gonzalez-Elipe, ARApplied Surface Science, 488 (2019) 791-800 DOI: 10.1016/j.apsusc.2019.05.273
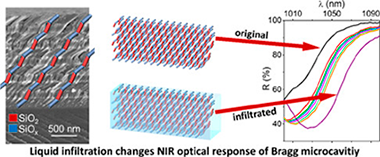
Abstract
Traditionally porous silicon based photonic structures have been prepared by electrochemically etching of silicon. In this work, porous multilayers of nanocolumnar SiOx and SiO2 thin films acting as near infrared (NIR) 1D-photonic nanostructures are prepared by magnetron sputtering deposition at oblique angles (MS-OA). Simultaneous control of porosity and stoichiometry of the stacked films is achieved by adjusting the deposition angle and oxygen partial pressure according to a parametric formula. This new methodologoy is proved for the synthesis of SiOx thin films with x close to 0.4, 0.8, 1.2, 1.6 and nanostructures varying from compact (at 0 degrees deposition angle) to highly porous and nanocolumnar (at 70 degrees and 85 degrees deposition angles). The strict control of composition, structure and nanostructure provided by this technique permits a fine tuning of the absorption edge and refraction index at 1500 nm of the porous films and their manufacturing in the form of SiOx-SiO2 porous multilayers acting as near infrared (NIR) 1D-photonic structures with well-defined optofluidic responses. Liquid tunable NIR Bragg mirrors and Bragg microcavities for liquid sensing applications are presented as proof of concept of the possibilities of this MS-OA manufacturing method as an alternative to the conventional electrochemical fabrication of silicon based photonic structures.
Septiembre, 2019 · DOI: 10.1016/j.apsusc.2019.05.273
Fotocatálisis Heterogénea: Aplicaciones
Extraordinary visible photocatalytic activity of a Co0.2Zn0.8O system studied in the Remazol BB oxidation
KarimTanji; J.A.Navio; Jamal Naja; M.C.Hidalgo; Abdellah Chaqroune; C.Jaramillo-Páez; Abdelhak KherbecheJournal of Photochemistry and Photobiology A: Chemistry, 382 (2019) 111877 DOI: 10.1016/j.jphotochem.2019.111877

Abstract
Nanoparticles of CoxZn1-xO system with a nominal composition of x=0.2 were synthesized by the Solution Combustion Method (SCM). Structural and morphological studies as well as the chemical composition of the material were widely investigated by different techniques. Photocatalytic activity under UV and Visible illumination was studied by means of the Remazol Brilliant Blue dye (RBB) oxidation reaction. The effect of different experimental parameters, such as the initial dye concentration, photocatalyst mass, pH or hydrogen peroxide concentration on the RBB discoloration under UV irradiation was studied. Optimal experimental conditions were found to be a photocatalyst mass of 1 g.L-1, dye concentration of 20 mg.L-1 and solution pH of 11. Hydrogen peroxide addition was found to have no effect in the photocatalytic behavior of the material in the range of concentration studied (0 to 6•10-4 M). The optimal parameters were chosen to investigate the degradation of RBB under UV-illumination and just visible illumination. It was observed that the UV-photocatalytic property of pristine ZnO for the RBB removal was scarcely improved after cobalt-incorporation, whereas the effect of cobalt incorporation into ZnO greatly enhanced the RBB conversion under visible illumination. Even more interesting is that, under same experimental conditions, the visible efficiency of the Co-ZnO system is the same that the one showed under UV illumination, i.e. the system does not loose efficiency when illuminated only with visible light.
Septiembre, 2019 · DOI: 10.1016/j.jphotochem.2019.111877
Materiales de Diseño para la Energía y Medioambiente
Insoluble and Thermostable Polyhydroxyesters From a Renewable Natural Occurring Polyhydroxylated Fatty Acid
Benitez, JJ; Guzman-Puyol, S; Cruz-Carrillo, MA; Ceseracciu, L; Moreno, AG; Heredia, A; Heredia-Guerrero, JAFrontiers in Chemistry, 7 (2019) art. 643 DOI: 10.3389/fchem.2019.00643
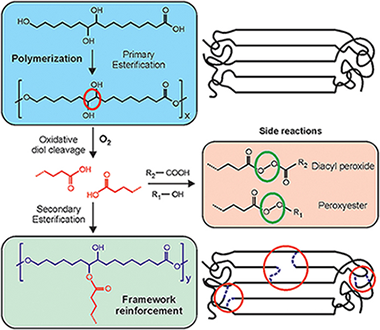
Abstract
To explore the potential of long chain polyhydroxyalkanoates as non-toxic food packaging materials, the characterization of polyesters prepared from a natural occurring polyhydroxylated C16 carboxylic acid (9,10,16-trihydroxyhexadecanoic or aleuritic acid) has been addressed. Such monomer has been selected to elucidate the reactivity of primary and secondary hydroxyl groups and their contribution to the structure and properties of the polyester. Resulting polyaleuritate films have been produced using an open mold in one-step, solvent-free self-polycondensation in melt state and directly in air to evaluate the effect of oxygen in their final physical and chemical properties. These polymers are amorphous, insoluble, and thermostable, being therefore suitable for solvent, and heat resistant barrier materials. Structurally, most of primary hydroxyls are involved in ester bonds, but there is some branching arising from the partial participation of secondary O-H groups. The oxidative cleavage of the vicinal diol moiety and a subsequent secondary esterification had a noticeable effect on the amorphization and stiffening of the polyester by branching and densification of the ester bond network. A derivation of such structural modification was the surface compaction and the reduction of permeability to water molecules. The addition of Ti(OiPr)(4) as a catalyst had a moderate effect, likely because of a poor diffusion within the melt, but noticeably accelerated both the secondary esterification and the oxidative processes. Primary esterification was a high conversion bulk reaction while oxidation and secondary esterification was restricted to nearby regions of the air exposed side of cast films. The reason was a progressive hindering of oxygen diffusion as the reaction progresses and a self-regulation of the altered layer growth. Despite such a reduced extent, the oxidized layer noticeably increased the UV-vis light blockage capacity. In general, characterized physical properties suggest a high potential of these polyaleuritate polyesters as food preserving materials.
Septiembre, 2019 · DOI: 10.3389/fchem.2019.00643
Química de Superficies y Catálisis
Montmorillonite-stabilized gold nanoparticles for nitrophenol reduction
Chenouf, M; Megias-Sayago, C; Ammari, F; Ivanova, S; Centeno, MA; Odriozola, JAComptes Rendus Chimie, 22 (2019) 621-627 DOI: 10.1016/j.crci.2019.07.005
Abstract
Two gold-based catalysts were obtained by Au chemical reduction of the HAuCl(4 )precursor. The resulting nanoparticles were stabilized and immobilized on montmorillonite (Mt) and montmorillonite-ceria (Mt/CeO2). All prepared catalysts were active in 4-nitrophenol to aminophenol reduction at room temperature. Synergy between montmorillonite and ceria is postulated in such a way that the montmorillonite phase hinders particle growth either by influencing the nucleation behavior of gold or by increasing the number of nucleation sites and raising the overall dispersion. The role of the ceria support, on the other hand, may be associated with the 4-NP adsorption at the ceria-gold interface, stabilizing the reaction intermediate and hence lowering the activation barrier for the reduction of 4-NP to 4-AP.
Septiembre, 2019 · DOI: 10.1016/j.crci.2019.07.005
Materiales de Diseño para la Energía y Medioambiente
Correlation of Structure and Performance of Hard Carbons as Anodes for Sodium Ion Batteries
Gomez-Martin, A; Martinez-Fernandez, J; Ruttert, M; Winter, M; Placke, T; Ramirez-Rico, JChemistry of Materials, 31 (2019) 7288-7299 DOI: 10.1021/acs.chemmater.9b01768

Abstract
Hard carbons are the material of choice as negative electrode in sodium ion batteries. Despite being extensively studied, there is still debate regarding the mechanisms responsible for storage in low- and high-potential regions. This work presents a comprehensive approach to elucidate the involved storage mechanisms when Na ions insert into such disordered structures. Synchrotron X-ray total scattering experiments were performed to access quantitative information on atomic ordering in these materials at the nanoscale. Results prove that hard carbons undergo an atomic rearrangement as the graphene layers cross-link at intermediate temperatures (1200-1600 degrees C), resulting in an increase of the average interplanar distance up to 1400 degrees C, followed by a progressive decrease. This increase correlates with the positive trend in the reversible capacity of biomass-derived carbons when processed up to 1200-1600 degrees C due to an increased capacity at low potential (<= 0.1 V vs Na/Na+). A decrease in achievable sloping capacity with increasing heat-treatment temperature arises from larger crystalline domains and a lower concentration of defects. The observed correlation between structural parameters and electrochemical properties clearly supports that the main storage of Na ions into a hard-carbon structure is based on an adsorption-intercalation mechanism.
Septiembre, 2019 · DOI: 10.1021/acs.chemmater.9b01768
Nanotecnología en Superficies y Plasma
Antibacterial Nanostructured Ti Coatings by Magnetron Sputtering: From Laboratory Scales to Industrial Reactors
Alvarez, R; Munoz-Pina, S; Gonzalez, MU; Izquierdo-Barba, I; Fernandez-Martinez, I; Rico, V; Arcos, D; Garcia-Valenzuela, A; Palmero, A; Vallet-Regi, M; Gonzalez-Elipe, AR; Garcia-Martin, JMNanomaterials, 9 (2019) art. 1217 DOI: 10.3390/nano9091217

Abstract
Based on an already tested laboratory procedure, a new magnetron sputtering methodology to simultaneously coat two-sides of large area implants (up to similar to 15 cm(2)) with Ti nanocolumns in industrial reactors has been developed. By analyzing the required growth conditions in a laboratory setup, a new geometry and methodology have been proposed and tested in a semi-industrial scale reactor. A bone plate (DePuy Synthes) and a pseudo-rectangular bone plate extracted from a patient were coated following the new methodology, obtaining that their osteoblast proliferation efficiency and antibacterial functionality were equivalent to the coatings grown in the laboratory reactor on small areas. In particular, two kinds of experiments were performed: Analysis of bacterial adhesion and biofilm formation, and osteoblasts-bacteria competitive in vitro growth scenarios. In all these cases, the coatings show an opposite behavior toward osteoblast and bacterial proliferation, demonstrating that the proposed methodology represents a valid approach for industrial production and practical application of nanostructured titanium coatings.
Septiembre, 2019 · DOI: 10.3390/nano9091217
Materiales para Bioingeniería y Regeneración Tisular
MTA HP Repair stimulates in vitro an homogeneous calcium phosphate phase coating deposition
Jiménez-Sánchez, M.D.C.; Segura-Egea, J.J.; Díaz-Cuenca, A.Journal of Clinical and Experimental Dentistry, 11 (2019) e322-e326 DOI: 10.4317/jced.55661

Abstract
Background: To study the mineralization capacity in vitro of the bioceramic endodontic material MTA HP Repair. Material and Methods: Bioactivity evaluation in vitro was carried out, by soaking processed cement disk in simulated body fluid (SBF) during 168 h. The cement surface was studied by Fourier transform infrared spectroscopy (FTIR), field emission gun scanning electron microscopy (FEG-SEM) and energy dispersive X-ray analysis (EDX). Release to the SBF media of ionic degradation products was monitored using inductively coupled plasma atomic emission spectroscopy (ICP-AES). Results: FT-IR showed increasing formation of phosphate phase bands at 1097, 960, 607 and 570 cm -1 with prolonged SBF soaking. FEG-SEM analysis reveals that HP produces a effectively surface covering consisting in homogeneous spherical phosphate phase aggregates with an average diameter of 0.5 -1 .0 μm. EDX analysis comparing un-treated (hydrated), 24 h and 72 h SBF treated surfaces of MTA HP Repair revealed phosphate deposition after 24 h, with high phosphorous/silicon element ratio signal measured after 24 h, indicating a very high phosphate phase deposition for this material. Conclusions: The study shows that MTA HP Repair produces a quick and effective bioactive response in vitro in terms of crystalline calcium phosphate surface coating formation. The high bioactive response of MTA HP Repair makes it an interesting candidate for endodontic use as repair cement.
Agosto, 2019 · DOI: 10.4317/jced.55661
Materiales para Bioingeniería y Regeneración Tisular
Physicochemical parameters - hydration performance relationship of the new endodontic cement MTA Repair HP
Jiménez-Sánchez, M.D.C.; Segura-Egea, J.J.; Díaz-Cuenca, A.Journal of Clinical and Experimental Dentistry, 11 (2019) e739-e744 DOI: 10.4317/jced.56013
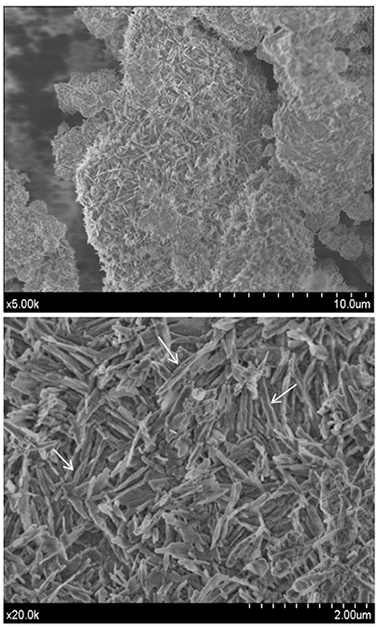
Abstract
Background: To characterize the chemical composition and textural parameters of the MTA Repair HP precursor powder and their influence to hydration performance. Material and Methods: Un-hydrated precursor material was characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), X-ray fluorescence (XRF), laser diffraction (LD), N2 physisorption and field emission gun scanning electron microscopy (FEG-SEM). Setting time was assessed according to ASTM specification C 266. Hydrated material was analysed by XRD, FT-IR, energy dispersive X-ray (EDX) analysis and FEG-SEM. Results: Ca3SiO5 and Ca2SiO4, in addition to CaWO4 as radiopacifier are the main compositional phases. Other measured parameters indicate high specific surface area of 4.8 m2 g-1, high aluminium content of 1.7 wt.% and low initial and final setting times of 12 and 199 min, respectively. Singular microstructural features consisting of high aspect ratio nanoparticles are main constituents of un-hydrated precursor. Besides, FEM-SEM observation shows notably growth of hexagonal shaped plate-like morphologies homogeneously distributed along the sample during hydration process. Conclusions: The short setting time measured for HP Repair, is correlated with high surface area of precursor powder, high Al content and the absence of compositional sulphate phases.
Agosto, 2019 · DOI: 10.4317/jced.56013
Materiales de Diseño para la Energía y Medioambiente
Bio-based composite fibers from pine essential oil and PLA/PBAT polymer blend. Morphological, physicochemical, thermal and mechanical characterization
Hernandez-Lopez, M; Correa-Pacheco, ZN; Bautista-Banos, S; Zavaleta-Avejar, L; Benitez-Jimenez, JJ; Sabino-Gutierrez, MA; Ortega-Gudino, PMaterials Chemistry and Physics, 234 (2019) 345-353 DOI: 10.1016/j.matchemphys.2019.01.034
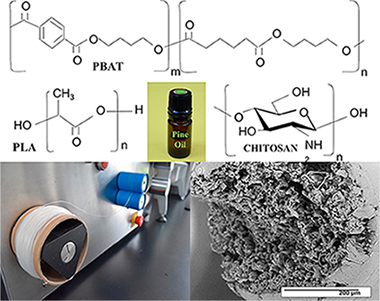
Abstract
Biodegradable aliphatic polyesters are an alternative to reduce the use of synthetic plastic materials that cause severe damage to the environment. Formulations based on poly (lactic acid) (PLA) and poly (butylene adipate-co-terephthalate) (PBAT), were mixed in a 60:40 ratio, adding different concentrations of pine essential oil through the use of extrusion technology to obtain biodegradable polymer fibers. Some formulations were coated with chitosan. All the elaborated fibers were characterized by Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy-Attenuated Total Reflection (FTIR-ATR), Differential Scanning Calorimetry (DSC), X-ray Diffraction (XRD) and mechanical properties. The SEM studies showed that the PBAT improves the tenacity and provides greater elasticity promoting the interaction between the blends phases with fibril formation. In the FTIR-ATR analysis, compatibility between the blends was observed due to a possible interaction of the carbonyl group of PBAT with PLA. The DSC and the mechanical properties showed partial miscibility of the blends, indicating, that the plasticizing action of the essential oil gave greater mobility, flexibility, less rigidity and crystallization in the blends. A lower Young's modulus and greater elongation at break was also observed.
Agosto, 2019 · DOI: 10.1016/j.matchemphys.2019.01.034
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
Support effects on NiO-based catalysts for the oxidative dehydrogenation (ODH) of ethane
Delgado, D; Sanchis, R; Cecilia, JA; Rodriguez-Castellon, E; Caballero, A; Solsona, B; Nieto, JMLCatalysis Today, 333 (2019) 10-16 DOI: 10.1016/j.cattod.2018.07.010

Abstract
We report on the effect of NiO-support interactions on the chemical nature of Ni species in a series of supported NiO catalysts for the ODH of ethane. SiO2, TiO2-anatase, a high surface area TiO2 and a porous clay hetero-structure (PCH) with TiO2 and SiO2 pillars were used as supports, which led to a selectivity to ethylene in the range 30-90% over supported NiO catalysts. The catalysts were characterized by means of XRD, N-2-Adsorption, H-2-TPR, XPS and in situ (under H-2 reductive atmosphere) and ex situ XAS spectroscopy. The catalytic performance of supported materials is discussed in terms of their reducibility and specific reduction kinetics, but also taking into account the specific chemical nature of Ni species on each catalyst. The influence of the particle size and the presence of Ni and O vacancies on the catalytic performance in the ODH of ethane is inferred.
Agosto, 2019 · DOI: 10.1016/j.cattod.2018.07.010
Química de Superficies y Catálisis
Au/Al2O3 - Efficient catalyst for 5-hydroxymethylfurfural oxidation to 2,5-furandicarboxylic acid
Megias-Sayago, C; Lolli, A; Ivanova, S; Albonetti, S; Cavani, F; Odriozola, JACatalysis Today, 333 (2019) 169-175 DOI: 10.1016/j.cattod.2018.04.024

Abstract
The catalytic activity of a simple Au/Al2O3 catalytic system prepared by the direct anionic exchange (DAE) method was evaluated in the selective 5-hydroxymethylfurfural (HMF) oxidation under mild conditions, using molecular oxygen as the oxidant. The influence of the HMF/NaOH ratio and reaction time on product yield and distribution were studied and discussed in detail. Extremely high activity and selectivity were observed in mild conditions, with 99% of 2,5-furandicarboxylic acid (FDCA) production at full HMF conversion after 4 h with the use of only 4 equivalents of NaOH at 70 degrees C. Catalyst viability and stability were verified by repeating the cycle up to five times. Changes in the nature of the support were also contemplated by introducing some ceria fraction, i.e. 20 wt%.
Agosto, 2019 · DOI: 10.1016/j.cattod.2018.04.024
Reactividad de Sólidos
Mechanically induced combustion synthesis and thermoelectric properties of nanostructured strontium hexaboride (SrB6)
Jalaly, M; Khosroshahi, BK; Gotor, FJ; Sayagues, MJ; Yamini, SA; Failamani, F; Mori, TCeramics International, 45 (2019) 14426-14431 DOI: 10.1016/j.ceramint.2019.04.163
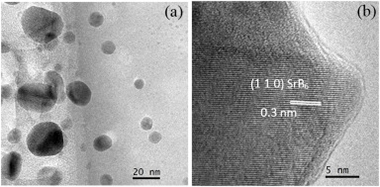
Abstract
The nanoparticles of strontium hexaboride (SrB6) were synthesized by a mechanically induced magnesiothermic combustion in the Mg/B2O3/SrO system. Ignition time in this system was recorded to be 23 min of milling. X-ray diffraction(XRD), X-ray photoelectron spectroscopy (XPS), and high-resolution transmission electron microscopy (HRTEM) techniques were used to characterize the combustion product. Thermal analysis was employed to assess the formation mechanism. It was revealed that Mg initially reduced B2O3 in a combustive manner to generate elemental boron and a large amount of heat, resulting in the reduction of SrO by Mg at high temperature. The in-situ formed elemental Sr and B react immediately to generate SrB6. Thermoelectric properties of consolidated SrB6, including thermal conductivity, Seebeck coefficient, electrical conductivity, and figure-of-merit were evaluated at the temperature range of 300–873 K.
Agosto, 2019 · DOI: 10.1016/j.ceramint.2019.04.163
Materiales Coloidales
Luminescence and X-ray Absorption Properties of Uniform Eu3+:(H3O)Lu3F10 Nanoprobes
Gonzalez-Mancebo, D; Becerro, AI; Corral, A; Balcerzyk, M; Ocana, MNanomaterials, 9 (2019) 1153 DOI: 10.3390/nano9081153

Abstract
Due to the high atomic number of lutetium and the low phonon energy of the fluoride matrix, Lu-based fluoride nanoparticles doped with active lanthanide ions are potential candidates as bioprobes in both X-ray computed tomography and luminescent imaging. This paper shows a method for the fabrication of uniform, water-dispersible Eu3+:(H3O)Lu3F10 nanoparticles doped with different Eu contents. Their luminescent properties were studied by means of excitation and emission spectra as well as decay curves. The X-ray attenuation capacity of the phosphor showing the highest emission intensity was subsequently analyzed and compared with a commercial contrast agent. The results indicated that the 10% Eu3+-doped (H3O)Lu3F10 nanoparticles fabricated with the proposed polyol-based method are good candidates to be used as dual probes for luminescent imaging and X-ray computed tomography.
Agosto, 2019 · DOI: 10.3390/nano9081153
Materiales de Diseño para la Energía y Medioambiente
Natural abundance O-17 MAS NMR and DFT simulations: New insights into the atomic structure of designed micas
Pavon, E; Osuna, FJ; Alba, MD; Delevoye, LSolid State Nuclear Magnetic Resonance, 100 (2019) 45-51 DOI: 10.1016/j.ssnmr.2019.03.006
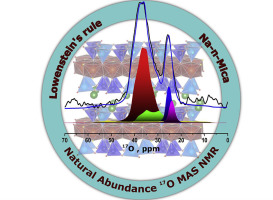
Abstract
Combining O-17 Magic-Angle Spinning (MAS) NMR at natural abundance with DFT calculations is a promising methodology to shed light on the structure and disorder in tetrahedral sheets of designed micas with enhanced properties. Among brittle micas, synthetic mica is an important alternative to natural ones with a swelling sheet-like structure that results in many applications, by exploiting unique characteristics. Lowenstein's rule is one of the main chemical factor that determines the atomic structure of aluminosilicates and furthermore their properties. In the present article, O-17 MAS NMR spectroscopy is used to validate (or not) the agreement of the Lowenstein's rule with the distribution of Si and Al sites in the tetrahedral sheets of synthetic micas. O-17 MAS spectra of synthetic high-charged micas exhibit two regions of signals that revealed two distinguishable oxygen environments, namely Si-O-X (with X = Si, Al-tet , Mg) and Al-tet -O-Y (Y=Mg or Al-tet). DFT calculations were also conducted to obtain the O-17 chemical shift and other NMR features like the quadrupolar coupling constant, C-Q, for all of the oxygen environments encountered in the two model structures, one respecting the Lowenstein's rule and the other involving Al-tet -O-Al-tet and Si-O-Si environments. Our DFT calculations support the O-17 assignment, by confirming that Al-tet -O-3Mg and Al tet -O-Al tet oxygen environments show chemical shifts under 30 ppm and more important, with quadrupolar coupling constants of about 1 MHz, in line with the spectral observation. By quantifying the O-17 MAS NMR spectra at natural abundance, we demonstrate that one of the synthetic mica compositions does not meet the Lowenstein's rule.
Agosto, 2019 · DOI: 10.1016/j.ssnmr.2019.03.006
Química de Superficies y Catálisis
Carbon Supported Gold Nanoparticles for the Catalytic Reduction of 4-Nitrophenol
Molina, HR; Munoz, JLS; Leal, MID; Reina, TR; Ivanova, S; Gallego, MNC; Odriozola, JAFrontiers in Chemistry, 7 (2019) 548 DOI: 10.3389/fchem.2019.00548
Abstract
This work is a detailed study on how to optimize gold colloids preparation and their deposition to very different in nature carbon materials. The change of the continuous phase and its dielectric constant is used to assure the good dispersion of the hydrophilic/hydrophobic carbons and the successful transfer of the preformed small size colloids to their surface. The sintering behavior of the particles during the calcination step is also studied and the optimal conditions to reduce to a minimum the particle size increase during the protecting agent removal phase are found. The as prepared catalysts have been tested in a relevant reaction in the field of environmental catalysis such as the reduction of 4-nitrophenol leading to promising results. Overall, this work proposes an important methodology to follow when a carbonaceous material are selected as catalyst supports for green chemistry reactions.
Agosto, 2019 · DOI: 10.3389/fchem.2019.00548
Materiales para Bioingeniería y Regeneración Tisular
Higher hydration performance and bioactive response of the new endodontic bioactive cement MTA HP repair compared with ProRoot MTA white and NeoMTA plus
Jimenez-Sanchez, Maria Del Carmen; Segura-Egea, Juan Jose; Diaz-Cuenca, AranzazuJournal of biomedical materials research. Part B, Applied biomaterials, 107 (2019) 2109-2120 DOI: 10.1002/jbm.b.34304

Abstract
The aim of this study was to characterize the hydration performance and the bioactive response of the new bioactive endodontic cement MTA HP repair (HP), comparing its physicochemical parameters with those of ProRoot MTA White (Pro) and NeoMTA Plus (Neo). Un-hydrated precursor materials were characterized by X-ray fluorescence, laser diffraction, N2 physisorption and field emission gun scanning electron microscopy (FEG-SEM). Setting time was assessed according to ASTM specification C 266. Hydrated materials were analyzed by X-ray diffraction, Fourier transform infrared spectroscopy (FT-IR) and (FEG-SEM). Bioactivity evaluation in vitro was carried out, by soaking processed cement disk in simulated body fluid (SBF) during 168 h. The cements surface was studied by FT-IR, FEG-SEM, and energy dispersive X-ray. Release to the SBF media of ionic degradation products was monitored using inductively coupled plasma atomic emission spectroscopy. HP showed shorter initial setting time compared to Pro and Neo and produce a quick and effective bioactive response in vitro in terms of phosphate phase surface coating formation. This higher bioactive response for HP is correlated with increasing calcium aluminate content, increasing surface area of un-hydrated powder precursor and the increasing release capacity of Si ionic products of the final hydrated product. The higher bioactive response of MTA HP repair highlights this material, as very interesting to further investigate its performance to improve the outcome of vital pulp therapy procedures.
Agosto, 2019 · DOI: 10.1002/jbm.b.34304
Química de Superficies y Catálisis
Noble Metal Supported on Activated Carbon for "Hydrogen Free" HDO Reactions: Exploring Economically Advantageous Routes for Biomass Valorisation
Jin, W; Santos, JL; Pastor-Perez, L; Gu, S; Centeno, MA; Reina, TRChemcatchem (2019) 4434-4441 DOI: 10.1002/cctc.201900841

Abstract
An innovative route for bio‐compounds upgrading via “hydrogen‐free” hydrodeoxygenation (HDO) is proposed and evaluated using guaiacol as a model compound in a high‐pressure batch reactor. Experimental results showed that noble metal supported on activated carbon catalysts are able to conduct tandem multiple steps including water splitting and subsequent HDO. The activity of Ru/C catalyst is superior to other studied catalysts (i. e. Au/C, Pd/C and Rh/C) in our water‐only HDO reaction system. The greater dispersion and smaller metal particle size confirmed by the TEM micrographs accounts for the better performance of Ru/C. This material also presents excellent levels of stability as demonstrated in multiple recyclability runs. Overall, the proposed novel approach confirmed the viability of oxygenated bio‐compounds upgrading in a water‐only reaction system suppressing the need of external H2 supply and can be rendered as a fundamental finding for the economical biomass valorisation to produce added value bio‐fuels.
Agosto, 2019 · DOI: 10.1002/cctc.201900841
Structural and compositional analysis of Co-based coatings after catalytic tests for the sodium borohydride hydrolysis
Beltran, AMMaterials Research Express, 6 (2019) art. 085511 DOI: 10.1088/2053-1591/ab1e27
Abstract
The use of Co-based catalysts for the sodium borohydride hydrolysis for hydrogen production is a well-known process as a source of clean energy, although its mechanisms are still under discussion. With the aim of acquiring a deeper knowledge about this catalytic process, three different catalysts (Co, CoC and CoB) were deposited as a thin film layer by magnetron sputtering onto a polymeric membrane, used as a substrate and analyzed by advance transmission and scanning-transmission electron microscopy techniques (STEM). Structural and compositional characterizations, by electron energy loss spectroscopy (EELS), have been performed on the coatings before and after their use as catalysts on the sodium borohydride reaction for 90 min, to check the production of hydrogen. Results have shown the formation of CoxB nanoflakes and other Co-based compounds over the catalysts and related to their catalytic activity. Knowing the changes in the structure and composition of the catalysts is key to understanding their catalytic behavior, activity and durability. Among the analyzed catalysts, the Co-C presents better activity during the first cycles, which is related to a larger formation of CoxB.
Agosto, 2019 · DOI: 10.1088/2053-1591/ab1e27
Química de Superficies y Catálisis - Reactividad de Sólidos
Influence of the preparation method in the metal-support interaction and reducibility of Ni-Mg-Al based catalysts for methane steam reforming
Azancot, L; Bobadilla, LF; Santos, JL; Cordoba, JM; Centeno, MA; Odriozola, JAInternational Journal of Hydrogen Energy, 44 (2019) 19827-19840 DOI: 10.1016/j.ijhydene.2019.05.167
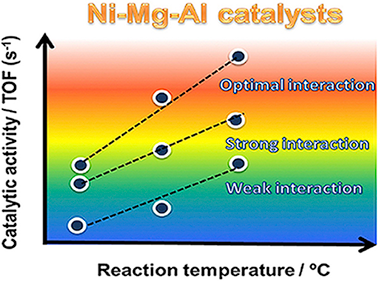
Abstract
Ni-Mg-Al based catalysts were prepared using different preparation methods (impregnation, impregnation-coprecipitation and coprecipitation) and tested in steam reforming of methane. The differences observed in catalytic activity were directly correlated to the physicochemical properties and the different degree of Ni-Mg-Al interaction. The reducibility results showed that the catalyst prepared by the impregnation-coprecipitation method presented the most optimal metal-support interaction to reduce the NiO preserving the Ni-0 particles highly dispersed on the support surface. These results demonstrate that the structure and catalytic performance of Ni-Mg-Al based catalysts can be tuned by controlling the metal-support interaction through of the preparation method.
Julio, 2019 · DOI: 10.1016/j.ijhydene.2019.05.167
Laboratory multi-technique study of Spanish decorated leather from the 12th to 14th centuries
Franquelo, ML; Duran, A; Perez-Rodriguez, JLSpectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 218 (2019) 331-341 DOI: 10.1016/j.saa.2019.04.012

Abstract
This work comprises an exhaustive study of Spanish decorative leathers dating from the 12th to 14th centuries. These paintings are considered a key example of a crucible of artistic styles: Gothic, Islamic and Florentine Trecento. The goal of this work was to use the scientific information provided by a number of experimental techniques – namely EDX, micro-FTIR, micro-Raman and micro-XRD – to assess the dating of the wooden vault, leather preparation and filling fibres. Another goal was to assess the artistic technique based on the characterization of pigments and the differentiation between original materials and those added throughout its history. Gypsum was the original preparation layer extended over the leather. A new preparation stratum was added in further interventions with the artwork. The original pictorial materials and those used during refurbishments have been identified. Original pigments were: red lead, Mars red, red lake, cinnabar, lapis lazuli, red ochres, raw sienna, white lead and charcoal black. Gilding was also found. Pigments added during restoration were: barite, emerald green, rutile, anatase, Mars red, cadmium red, lithopone, cadmium yellow, charcoal black and orpiment.
Julio, 2019 · DOI: 10.1016/j.saa.2019.04.012
Nanotecnología en Superficies y Plasma
Plasma Enabled Conformal and Damage Free Encapsulation of Fragile Molecular Matter: from Surface-Supported to On-Device Nanostructures
Alcaire, M; Aparicio, FJ; Obrero, J; Lopez-Santos, C; Garcia-Garcia, FJ; Sanchez-Valencia, JR; Frutos, F; Ostrikov, K; Borras, A; Barranco, AAdvanced Functional Materials, (2019) art. 1903535 DOI: 10.1002/adfm.201903535
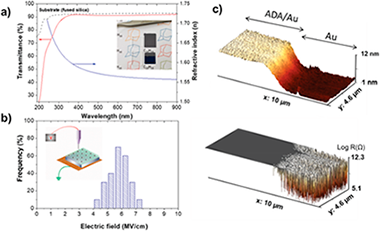
Abstract
Damage-free encapsulation of molecular structures with functional nanolayers is crucial to protect nanodevices from environmental exposure. With nanoscale electronic, optoelectronic, photonic, sensing, and other nanodevices based on atomically thin and fragile organic matter shrinking in size, it becomes increasingly challenging to develop nanoencapsulation that is simultaneously conformal at atomic scale and does not damage fragile molecular networks, while delivering added device functionality. This work presents an effective, plasma-enabled, potentially universal approach to produce highly conformal multifunctional organic films to encapsulate atomically thin graphene layers and metalorganic nanowires, without affecting their molecular structure and atomic bonding. Deposition of adamantane precursor and gentle remote plasma chemical vapor deposition are synergized to assemble molecular fragments and cage-like building blocks and completely encapsulate not only the molecular structures, but also the growth substrates and device elements upon nanowire integration. The films are insulating, transparent, and conformal at sub-nanometer scale even on near-tip high-curvature areas of high-aspect-ratio nanowires. The encapsulated structures are multifunctional and provide effective electric isolation, chemical and environmental protection, and transparency in the near-UV-visible-near-infrared range. This single-step, solvent-free remote-plasma approach preserves and guides molecular building blocks thus opening new avenues for precise, atomically conformal nanofabrication of fragile nanoscale matter with multiple functionalities.
Julio, 2019 · DOI: 10.1002/adfm.201903535
Química de Superficies y Catálisis
Au/CeO2-ZnO/Al2O3 as Versatile Catalysts for Oxidation Reactions: Application in Gas/Liquid Environmental Processes
Megias-Sayago, C; Reina, TR; Ivanova, S; Odriozola, JAFrontiers in Chemistry, 7 (2019) art. 504 DOI: 10.3389/fchem.2019.00504

Abstract
The present work showcases the versatility of nanogold systems supported on Zn-doped ceria when applied in two important environmental processes, the total CO oxidation, and the liquid phase oxidation of glucose to gluconic acid. In the CO oxidation the suitability of these materials is clearly demonstrated achieving full conversions even at sub-ambient conditions. Regarding the glucose oxidation our materials display high conversion values (always over 50%) and very importantly full or almost full selectivity toward gluconic acid-an added value platform chemical in the context of biomass upgrading routes. The key factors controlling the successful performance on both reactions are carefully discussed and compared to previous studies in literature. To our knowledge this is one of the very few works in catalysis by gold combining liquid and gas phase reactions and represents a step forward in the flexible behavior of nano gold catalysts.
Julio, 2019 · DOI: 10.3389/fchem.2019.00504
Nanotecnología en Superficies y Plasma
Large gap atmospheric pressure barrier discharges using ferroelectric materials
Navascues, P.; Gonzalez-Elipe, A. R.; Cotrino, J.; Gomez-Ramirez, A.Plasma Sources Sciences & Tecnology, 28 (2019) 075002 DOI: 10.1088/1361-6595/ab28ce
Abstract
This work reports a phenomenological comparative study of atmospheric pressure barrier plasmas using ferroelectric (ferroelectric barrier discharge (FBD)) and dielectric (dielectric barrier discharge (DBD)) plates to moderate the discharge. For FBD operation and large inter-electrode distances, experiments with helium carried out in a parallel plate reactor as a function of applied voltage have shown an enhancement of one order of magnitude in the charge transferred through the circuit. In a similar way to DBDs, FBDs rendered a laterally localized arrangement of discrete columnar discharges with a pattern distribution and an overall current intensity that depended on operation conditions. However, unlike the regular columnar pattern found for DBD operation, discharge columns in the FBD mode appear randomly and inhomogeneously distributed on the ferroelectric surface. This geometrical behavior of FBD plasma columns, as well as the singular variation of current with applied voltage and the particular shape characteristics of the current discharge curves have been accounted for by the high capacity of ferroelectric surfaces to randomly accumulate charge and to promote the emission of secondary electrons in the presence of a plasma.
Julio, 2019 · DOI: 10.1088/1361-6595/ab28ce
Reactividad de Sólidos
Effects of Boron Addition on the Microstructure and Mechanical Properties of (Ti,Ta)(C,N)-Co Based Cermets
Chicardi, E; Martinez, FJGMetals, 9 (2019) art. 787 DOI: 10.3390/met9070787
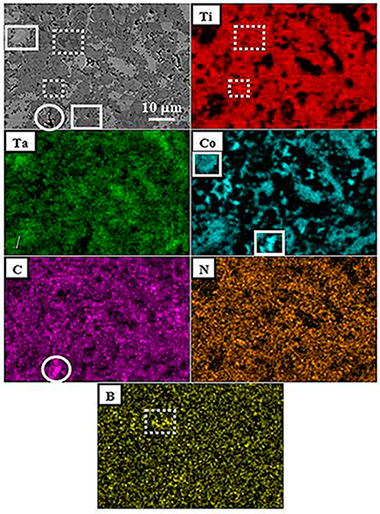
Abstract
In this work, a titanium-tantalum carbonitride based cermet, with cobalt as the binder phase and boron as a sintering additive, was developed by a mechanically induced self-sustaining reaction process using two different methodologies. The boron additive was added to prevent the formation of brittle intermetallic compounds generally formed during the liquid phase sintering step due to the excessive ceramic dissolution into the molten binder phase. A systematic study was carried out to understand the effects of boron addition on the nature of the phases, microstructure, and mechanical properties of cermets. With the boron addition, the formation of two different boride solid solutions, i. e., (Ti, Ta)B-2 and (Ti, Ta)(3)B-4, was observed. Moreover, the nature of the binder was also modified, from the (Ti, Ta)Co-2 brittle intermetallic compound (for cermets without boron addition) to ductile and tough (Ti, Ta)Co-3 and alpha-Co phases (for cermets with boron addition). These modifications caused, as a general trend, the increase of hardness and toughness in cermets.
Julio, 2019 · DOI: 10.3390/met9070787
Propiedades mecánicas, modelización y caracterización de cerámicos avanzados
Does grain size have an influence on intrinsic mechanical properties and conduction mechanism of near fully-dense boron carbide ceramics?
Moshtaghioun, BM; Laguna-Bercero, MA; Gomez-Garcia, D; Pena, JIJournal of Alloys and Compounds, 795 (2019) 408-415 DOI: 10.1016/j.jallcom.2019.05.037
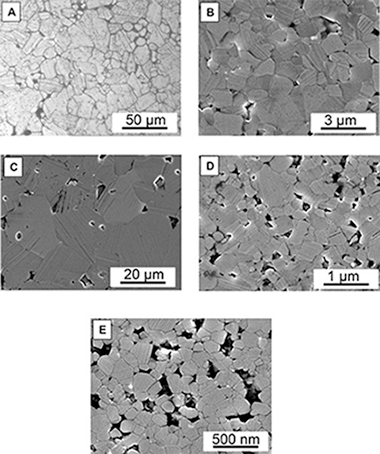
Abstract
This work is concentrated on getting a reply to the following question: how does the grain size of boron carbide specimens influence on their mechanical and electrical response? It is a common issue that both essential properties are usually affected by the grain boundaries. To this purpose, a set of near fully-dense boron carbide specimens were prepared by spark plasma sintering. In order to reduce residual porosity and grain-size effects, nanoindentation tests at room temperature were conducted. DC conductivity was measured through four-point test technique from room temperature up to 800 °C. The results show that hardness can reach values as high as ∼60 GPa and plasticity onset takes place at around 23 GPa by dislocation nucleation. Regarding the conductivity, it is found that grain boundaries can block the mobility of bipolarons in an effective way. A simple additive law is provided to account for the resistivity of boron carbide polycrystals.
Julio, 2019 · DOI: 10.1016/j.jallcom.2019.05.037
Materiales de Diseño para la Energía y Medioambiente
Bionanocomposites based on chitosan intercalation in designed swelling high-charged micas
Alba, MD; Cota, A; Osuna, FJ; Pavon, E; Perdigon, AC; Raffin, FScientific Reports, 9 (2019) art. 10265 DOI: 10.1038/s41598-019-46495-z
Abstract
Bionanocomposites based on layered inorganic components, as clays, and polymers of biological origin, as chitosan, have a major impact in medical and environmental fields, being economical and environmentally friendly materials. Na-Mn micas (n = 2 and 4) with controlled surface charge, high cation exchange capacity and swelling behaviour, are attractive inorganic composite components that exhibit improved adsorption properties compared to other inorganic solids which makes them potentially useful for bionanocomposites. The goal of this research was to explore the potential use of those synthetic brittle micas to form eco-friendly bionanocomposites with chitosan biopolymer. Hence, chitosan-mica bionanocomposites were prepared by ion-exchange reaction between chitosan solution and synthetic high charge mica. X-ray diffraction, Fourier transform infrared spectroscopy, thermal analysis, MAS-NMR spectroscopy and zeta-potential have been employed for bionanocomposites characterization. The results showed that the adsorption of chitosan is effective, although a chitosan portion remains in the outer surface being hydrogen-bonded to the tetrahedral sheet of the silicate.
Julio, 2019 · DOI: 10.1038/s41598-019-46495-z
Nanotecnología en Superficies y Plasma
On‐Surface Synthesis and Characterization of Acene‐Based Nanoribbons Incorporating Four‐Membered Rings
Sanchez-Sanchez, C; Dienel, T; Nicolai, A; Kharche, N; Liang, LB; Daniels, C; Meunier, V; Liu, JZ; Feng, XL; Mullen, K; Sanchez-Valencia, JR; Groning, O; Ruffieux, P; Fasel, RChemistry-A European Journal DOI: 10.1002/chem.201901410
Abstract
A bottom up method for the synthesis of unique tetracene-based nanoribbons, which incorporate cyclobutadiene moieties as linkers between the acene segments, is reported. These structures were achieved through the formal [2+2] cycloaddition reaction of ortho-functionalized tetracene precursor monomers. The formation mechanism and the electronic and magnetic properties of these nanoribbons were comprehensively studied by means of a multitechnique approach. Ultra-high vacuum scanning tunneling microscopy showed the occurrence of metal-coordinated nanostructures at room temperature and their evolution into nanoribbons through formal [2+2] cycloaddition at 475 K. Frequency-shift non-contact atomic force microscopy images clearly proved the presence of bridging cyclobutadiene moieties upon covalent coupling of activated tetracene molecules. Insight into the electronic and vibrational properties of the so-formed ribbons was obtained by scanning tunneling microscopy, Raman spectroscopy, and theoretical calculations. Magnetic properties were addressed from a computational point of view, allowing us to propose promising candidates to magnetic acene-based ribbons incorporating four-membered rings. The reported findings will increase the understanding and availability of new graphene-based nanoribbons with high potential in future spintronics.
Julio, 2019 · DOI: 10.1002/chem.201901410
Química de Superficies y Catálisis
Size-tailored Ru nanoparticles deposited over gamma-Al2O3 for the CO2 methanation reaction
Navarro-Jaen, S; Navarro, JC; Bobadilla, LF; Centeno, MA; Laguna, OH; Odriozola, JAApplied Surface Science, 483 (2019) 750-761 DOI: 10.1016/j.apsusc.2019.03.248
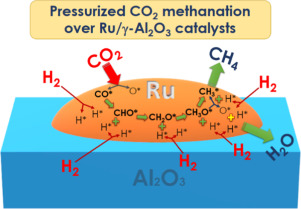
Abstract
By means of the polyol method, a series of 5 wt% Ru/Al2O3 catalysts was synthesized controlling the particle size of the ruthenium species. The physico-chemical characterization demonstrated the successful particle size control of the Ru species, in such a way that higher the Ru/PVP ratio, higher the Ru particle size. Moreover, there are evidences that suggest preferential growth of the RuO2 clusters depending on the Ru/PVP ratio. Regarding the catalytic activity during the CO2 methanation, the total conversion and the CH4 yield increased with the particle size of Ru. Nevertheless, a considerable enhancement of the catalytic performance of the most active system was evidenced at 4 bar, demonstrating the improvement of the thermodynamics (superior total conversion) and kinetics (superior reaction rate) of the CO2 methanation at pressures above the atmospheric one. Finally, the in situ DRIFTS study allowed to establish that CO2 was dissociated to CO* and O* species on the metallic Ru particles, followed by the consecutive hydrogenation of CO* towards CHO*, CH2O*, CH3O*, and finally CH4 molecules, which were further desorbed from the catalyst. Thus from the mechanistic point of view, a suitable particle size of the Ru nanoparticles along with the high-pressure effects results in the enhancement of the availability of hydrogen and consequently in the formation of CHxO species that enhance the cleavage of the C-O bond, which is the rate-determining step of the overall CO2 methanation process.
Julio, 2019 · DOI: 10.1016/j.apsusc.2019.03.248
Fotocatálisis Heterogénea: Aplicaciones
Preparation, characterization and photocatalytic degradation of Rhodamine B dye over a novel Zn3(PO4)2/BiPO4 catalyst
Naciri,Y.;Chennah,A.;Jaramillo-Páez,C.;Navío,J.A.;Bakiz, B.;Taoufyq,A.;Ezahri,M.;Villain,S.;Guinneton,F.;Benlhachemi,A.Journal of Environmental Chemical Engineering, 7 (2019) 103075 DOI: 10.1016/j.jece.2019.103075

Abstract
In this work, a facile method was used to synthesize the Zn3(PO4)2/BiPO4 composite photocatalysts with different Bi contents followed by heat treatment at 900 °C for 3 h. The as-prepared samples were studied by a variety of characterization techniques including X-ray diffraction (XRD), Scanning Electron Microscopy (SEM) combined with energy dispersive X-ray diffraction (EDX), Transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS) and UV–vis diffuse reflectance spectroscopy (DRS). The UV–vis spectroscopy was used to analyze the evolution of Rhodamine B discoloration in presence of the synthesized phosphate photocatalysts. The XRD, SEM-EDX, TEM, DRS and XPS analyses confirmed the formation of heterojunction structure between both materials, during the process of co-precipitation and ulterior heat treatment. The photocatalytic tests showed that photocatalytic ability of the 70% Bi-Zn3(PO4)2 composites was higher than that of pure Zn3(PO4)2 and BiPO4 after 1 h of UV-illumination. The obviously enhanced photocatalytic activity of the 70% Bi-Zn3(PO4)2 sample could be mainly attributed to the formation of the heterojunction, accelerating the separation of photogenerated charge carriers. A plausible mechanism of the photocatalytic degradation of RhB on Zn3(PO4)2/BiPO4 composites is proposed. The reduction in the Chemical Oxygen Demand (COD) revealed the mineralization of dye along with color removal. Thus, it can be suggested that the 70% Bi-Zn3(PO4)2 can serve as a promising photocatalyst in the degradation of organic contaminants under UV light.
Junio, 2019 · DOI: 10.1016/j.jece.2019.103075
Nanotecnología en Superficies y Plasma
Multifunctional antimicrobial chlorhexidine polymers by remote plasma assisted vacuum deposition
Mora-Boza, A; Aparicio, FJ; Alcaire, M; Lopez-Santos, C; Espinos, JP; Torres-Lagares, D; Borras, A; Barranco, AFrontiers of chemical science and engineering, 13 (2019) 330-339 DOI: 10.1007/s11705-019-1803-6

Abstract
Novel antibacterial materials for implants and medical instruments are essential to develop practical strategies to stop the spread of healthcare associated infections. This study presents the synthesis of multifunctional antibacterial nanocoatings on polydimethylsiloxane (PDMS) by remote plasma assisted deposition of sublimated chlorhexidine powders at low pressure and room temperature. The obtained materials present effective antibacterial activity against Escherichia coli K12, either by contact killing and antibacterial adhesion or by biocide agents release depending on the synthetic parameters. In addition, these multifunctional coatings allow the endure hydrophilization of the hydrophobic PDMS surface, thereby improving their biocompatibility. Importantly, cell-viability tests conducted on these materials also prove their non-cytotoxicity, opening a way for the integration of this type of functional plasma films in biomedical devices.
Junio, 2019 · DOI: 10.1007/s11705-019-1803-6
Nanotecnología en Superficies y Plasma
2D compositional self-patterning in magnetron sputtered thin films
Garcia-Valenzuela, A; Alvarez, R; Rico, V; Espinos, JP; Lopez-Santos, MC; Solis, J; Siegel, J; del Campo, A; Palmero, A; Gonzalez-Elipe, ARApplied Surface Science, 480 (2019) 115-121 DOI: 10.1016/j.apsusc.2019.02.206
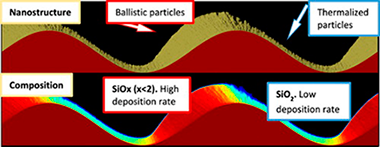
Abstract
Unlike topography patterning, widely used for numerous applications and produced by means of different technologies, there are no simple procedures to achieve surface compositional patterning at nanometric scales. In this work we have developed a simple method for 2D patterning the composition of thin films. The method relies on the magnetron sputtering deposition at oblique angles onto patterned substrates made by laser induced periodic surface structures (LIPSS). The method feasibility has been demonstrated by depositing SiOx thin films onto LIPSS structures generated in Cr layers. A heterogeneous and aligned distribution of O/Si ratios (and different Sin+ chemical states) along the LIPSS structure in length scales of some hundreds nm's has been proven by angle resolved X-ray photoelectron spectroscopy and a patterned arrangement of composition monitored by atomic force microscopy-Raman analysis. The obtained results are explained by the predictions of a Monte Carlo simulation of this deposition process and open the way for the tailored one-step fabrication of surface devices with patterned compositions.
Junio, 2019 · DOI: 10.1016/j.apsusc.2019.02.206
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
Comprehensive Experimental and Theoretical Study of the CO plus NO Reaction Catalyzed by Au/Ni Nanoparticles
Kyriakou, G; Marquez, AM; Holgado, JP; Taylor, MJ; Wheatley, AEH; Mehta, JP; Sanz, JF; Beaumont, SK; Lambert, RMACS Catalysis, 9 (2019) 4919-4929 DOI: 10.1021/acscatal.8b05154
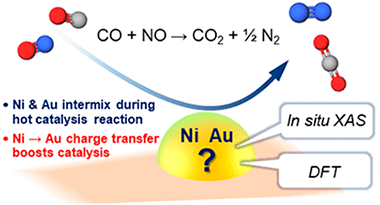
Abstract
The catalytic and structural properties of five different nanoparticle catalysts with varying Au/Ni composition were studied by six different methods, including in situ X-ray absorption spectroscopy and density functional theory (DFT) calculations. The as-prepared materials contained substantial amounts of residual capping agent arising from the commonly used synthetic procedure. Thorough removal of this material by oxidation was essential for the acquisition of valid catalytic data. All catalysts were highly selective toward N-2 formation, with 50-50 Au:Ni material being best of all. In situ X-ray absorption near edge structure spectroscopy showed that although Au acted to moderate the oxidation state of Ni, there was no clear correlation between catalytic activity and nickel oxidation state. However, in situ extended X-ray absorption fine structure spectroscopy showed a good correlation between Au Ni coordination number (highest for Ni50Au50) and catalytic activity. Importantly, these measurements also demonstrated substantial and reversible Au/Ni intermixing as a function of temperature between 550 degrees C (reaction temperature) and 150 degrees C, underlining the importance of in situ methods to the correct interpretation of reaction data. DFT calculations on smooth, stepped, monometallic and bimetallic surfaces showed that N + N recombination rather than NO dissociation was always rate-determining and that the activation barrier to recombination reaction decreased with increased Au content, thus accounting for the experimental observations. Across the entire composition range, the oxidation state of Ni did not correlate with activity, in disagreement with earlier work, and theory showed that NiO itself should be catalytically inert. Au-Ni interactions were of paramount importance in promoting N + N recombination, the rate-limiting step.
Junio, 2019 · DOI: 10.1021/acscatal.8b05154
Nanotecnología en Superficies y Plasma - Materiales Nanoestructurados y Microestructura
Exchange bias and two steps magnetization reversal in porous Co/CoO layer
Ovejero, JG; Godinho, V; Lacroix, B; Garcia, MA; Hernando, A; Fernandez, AMaterials & Design, 171 (2019) 107691 DOI: 10.1016/j.matdes.2019.107691
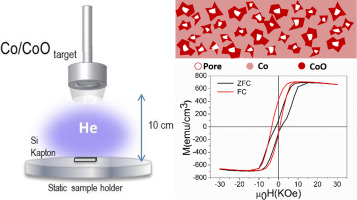
Abstract
In this paper Co/CoO thick layers (hundreds of nanometers) of different porosity and oxidation degree were prepared in a magnetron sputtering deposition processby tailoring the DC sputtering power, as well as the process gas and target composition. The control of the synthesis parameters allowed the nanostructuration of the films with a singular distribution of closed pores and a controlled amount of CoO. We observed an exchange bias field of 2.8 KOe for porous Co/CoO composites, similar to Co/CoO bilayers but for coatings thicker than 300 nm. Besides, it was observed that the coating presents bistable magnetic features when cooled under zero field conditions as a result of the unusual exchange coupling.
Junio, 2019 · DOI: 10.1016/j.matdes.2019.107691
2018
2018
Propiedades mecánicas, modelización y caracterización de cerámicos avanzados
Elusive super-hard B6C accessible through the laser-floating zone method
Moshtaghioun, BM; Cumbrera, FL; Gomez-Garcia, D; Pena, JIScientific Reports, 9 (2019) art. 13340 DOI: 10.1038/s41598-019-49985-2
Abstract
Boron carbide is among the most promising ceramic materials nowadays: their mechanical properties are outstanding, and they open potential critical applications in near future. Since sinterability is the most critical drawback to this goal, innovative and competitive sintering procedures are attractive research topics in the science and technology of this carbide. This work reports the pioneer use of the laser-floating zone technique with this carbide. Crystallographic, microstructural and mechanical characterization of the so-prepared samples is carefully analysed. One unexpected output is the fabrication of a B6C composite when critical conditions of growth rate are adopted. Since this is one of the hardest materials in Nature and it is achievable only under extremely high pressures and temperatures in hot-pressing, the use of this technique offers a promising alternative for the fabrication. Hardness and elastic modulus of this material reached to 52 GPa and 600 GPa respectively, which is close to theoretical predictions reported in literature.
Septiembre, 2019 · DOI: 10.1038/s41598-019-49985-2
- ‹ anterior
- 13 of 37
- siguiente ›














