Artículos SCI
2016
2016
Química de Superficies y Catálisis
Au/CeO2 Catalysts: Structure and CO Oxidation Activity
Centeno, MA; Reina, TR; Ivanova, S; Laguna, OH; Odriozola, JACatalysts, 6 (2016) 158
Show abstract ▽
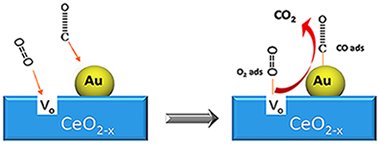
In this comprehensive review, the main aspects of using Au/CeO2 catalysts in oxidation reactions are considered. The influence of the preparation methods and synthetic parameters, as well as the characteristics of the ceria support (presence of doping cations, oxygen vacancies concentration, surface area, redox properties, etc.) in the dispersion and chemical state of gold are revised. The proposed review provides a detailed analysis of the literature data concerning the state of the art and the applications of gold–ceria systems in oxidation reactions.
Octubre, 2016 | DOI: 10.3390/catal6100158
Nanotecnología en Superficies y Plasma
Stabilization of catalyst particles against sintering on oxide supports with high oxygen ion lability exemplified by Ir-catalyzed decomposition of N2O
Yentekakis, IV; Goula, G; Panagiotopoulou, P; Kampouri, S; Taylor, MJ; Kyriakou, G; Lambert, RMApplied Catalysis B-Environmental, 192 (2016) 357-364
Show abstract ▽
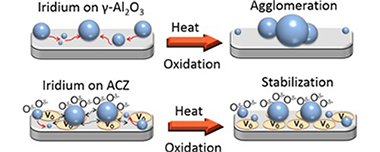
Iridium nanoparticles deposited on a variety of surfaces exhibited thermal sintering characteristics that were very strongly correlated with the lability of lattice oxygen in the supporting oxide materials. Specifically, the higher the lability of oxygen ions in the support, the greater the resistance of the nanoparticles to sintering in an oxidative environment. Thus with gamma-Al2O3 as the support, rapid and extensive sintering occurred. In striking contrast, when supported on gadolinia-ceria and alumina-ceria-zirconia composite, the Ir nanoparticles underwent negligible sintering. In keeping with this trend, the behavior found with yttria-stabilized zirconia was an intermediate between the two extremes. This resistance, or lack of resistance, to sintering is considered in terms of oxygen spillover from support to nanoparticles and discussed with respect to the alternative mechanisms of Ostwald ripening versus nanoparticle diffusion. Activity towards the decomposition of N2O, a reaction that displays pronounced sensitivity to catalyst particle size (large particles more active than small particles), was used to confirm that catalytic behavior was consistent with the independently measured sintering characteristics. It was found that the nanoparticle active phase was Ir oxide, which is metallic, possibly present as a capping layer. Moreover, observed turnover frequencies indicated that catalyst-support interactions were important in the cases of the sinter-resistant systems, an effect that may itself be linked to the phenomena that gave rise to materials with a strong resistance to nanoparticle sintering.
Septiembre, 2016 | DOI: 10.1016/j.apcatb.2016.04.011
Nanotecnología en Superficies y Plasma
Nanocolumnar association and domain formation in porous thin films grown by evaporation at oblique angles
Lopez-Santos, C; Alvarez, R; Garcia-Valenzuela, A; Rico, V; Loeffler, M; Gonzalez-Elipe, AR; Palmero, ANanotechnology, 27 (2016) 395702
Show abstract ▽
Porous thin films grown at oblique angles by evaporation techniques are formed by tilted nanocolumnar structures which, depending on the material type and growth conditions, associate along certain preferential directions, giving rise to large domains. This arrangement, commonly denoted as bundling association, is investigated in the present work by performing fundamental experiments and growth simulations. It is proved that trapping processes of vapor species at the film surface, together with the shadowing mechanism, mediate the anisotropic widening of the nanocolumns and promote their preferential coalescence along certain directions, giving rise to domains with different shape and size. The role of these two processes is thoroughly studied in connection with the formation of these domains in materials as different as SiO2 and TiO2.
Septiembre, 2016 | DOI: 10.1088/0957-4484/27/39/395702
Nanotecnología en Superficies y Plasma
Laser Treatment of Nanoparticulated Metal Thin Films for Ceramic Tile Decoration
Rico, VJ; Lahoz, R; Rey-Garcia, F; Yubero, F; Espinos, JP; de la Fuente, GF; Gonzalez-Elipe, ARApplied Materials & Interfaces, 8 (2016) 24880-24886
Show abstract ▽
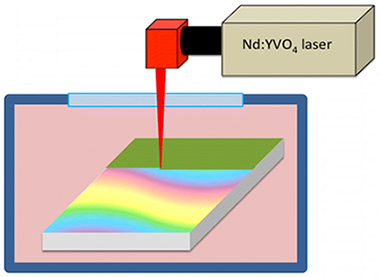
This paper presents a new method for the fabrication of metal-like decorative layers on glazed ceramic tiles. It consists of the laser treatment of Cu thin films prepared by electron-beam evaporation at glancing angles. A thin film of discontinuous Cu nanoparticles was electron-beam-evaporated in an oblique angle configuration onto ceramic tiles and an ample palette of colors obtained by laser treatment both in air and in vacuum. Scanning electron microscopy along with UV–vis–near-IR spectroscopy and time-of-flight secondary ion mass spectrometry analysis were used to characterize the differently colored layers. On the basis of these analyses, color development has been accounted for by a simple model considering surface melting phenomena and different microstructural and chemical transformations of the outmost surface layers of the samples.
Septiembre, 2016 | DOI: 10.1021/acsami.6b07469
Materiales Avanzados
Preparation of calcium carbonate as nanoparticles from inorganic precursors and sucrose as additive with potential application as biomaterial
Takabait, F; Mahtout, L; Villarejo, LP; Hurtado, BC; Soto, PJSBoletin de la Sociedad Española de Cerámcia y Vidrio, 55 (2016) 179-184
Show abstract ▽
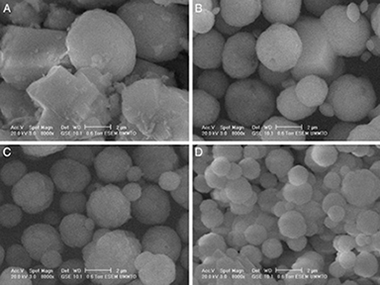
En esta comunicación se presentan unos primeros resultados de interés relevante sobre la obtención de carbonato de calcio precipitado como nanopartículas de los polimorfos vaterita y calcita. Se parte de precursores inorgánicos, nitrato de calcio tetrahidratado y bicarbonato de sodio, en presencia de sacarosa empleada como aditivo orgánico en disolución acuosa. Las fases cristalinas formadas se estudian mediante difracción de rayos X con un método cuantitativo y la morfología de las partículas obtenidas, mediante microscopia electrónica de barrido. Cuando no se emplea el aditivo orgánico se consigue la precipitación de calcita, polimorfo más estable termodinámicamente, como fase nanocristalina predominante (83%) mezclada con vaterita. Con una alta concentración del aditivo (67%) se obtiene vaterita como fase mayoritaria (> 98%). La utilización del aditivo en distinta proporción produce la formación de los 2 polimorfos de carbonato de calcio, siendo vaterita la fase predominante. La morfología de las partículas obtenidas muestra la formación de partículas nanoesféricas uniformes con contornos irregulares que se asocian a vaterita, así como partículas romboédricas de calcita cuando está presente, con potencial interés por su biocompatibilidad para su aplicación como biomateriales en implantes óseos.
Septiembre, 2016 | DOI: 10.1016/j.bsecv.2016.01.006
- ‹ anterior
- 212 of 420
- siguiente ›














