Artículos SCI
2015
2015
Propiedades mecánicas, modelización y caracterización de cerámicos avanzados
Ultra-fast and energy-efficient sintering of ceramics by electric current concentration
Zapata-Solvas, E; Gomez-Garcia, D; Dominguez-Rodriguez, A; Todd, RIScientific Reports, 5 (2015) art n. 8513
Show abstract ▽
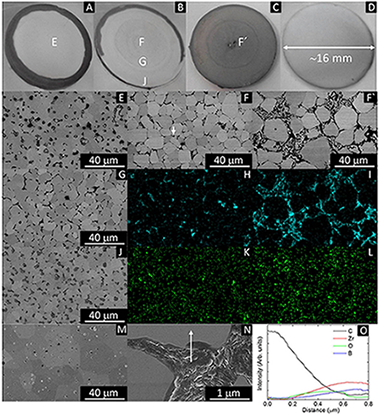
Electric current activated/assisted sintering (ECAS) techniques, such as electrical discharge sintering (EDS) or resistive sintering (RS), have been intensively investigated for longer than 50 years. In this work, a novel system including an electrically insulated graphite die for Spark Plasma Sintering (SPS) is described, which allows the sintering of any refractory ceramic material in less than 1 minute starting from room temperature with heating rates higher than 2000°C/min and an energy consumption up to 100 times lower than with SPS. The system alternates or combines direct resistive sintering (DRS) and indirect resistive sintering (IRS). Electrical insulation of the die has been achieved through the insertion of a film made of alumina fibers between the graphite die and the graphite punches, which are protected from the alumina fiber film by a graphite foil. This system localized the electric current directly through the sample (conductive materials) as in DRS and EDS, or through the thin graphite foil (non-conductive materials) as in IRS, and is the first system capable of being used under EDS or RS conditions independently combining current concentration/localization phenomena.
Febrero, 2015 | DOI: 10.1038/srep08513
Reactividad de Sólidos
Ca-looping for postcombustion CO2 capture: A comparative analysis on the performances of dolomite and limestone
Valverde, JM; Sanchez-Jimenez, PE; Perez-Maqueda, LAApplied Energy, 138 (2015) 202-215
Show abstract ▽

The low cost and wide availability of natural limestone (CaCO3) is at the basis of the industrial competitiveness of the Ca-looping (CaL) technology for postcombustion CO2 capture as already demonstrated by similar to 1 Mw(t) scale pilot projects. A major focus of studies oriented towards further improving the efficiency of the CaL technology is how to prevent the gradual loss of capture capacity of limestone derived CaO as the number of carbonation/calcination cycles is increased. Natural dolomite (MgCa(CO3)(2)) has been proposed as an alternative sorbent precursor to limestone. Yet, carbonation of MgO is not thermodynamically favorable at CaL conditions, which may hinder the capture performance of dolomite. In the work described in this paper we carried out a thermogravimetric analysis on the multicyclic capture performance of natural dolomite under realistic regeneration conditions necessarily implying high calcination temperature, high CO2 concentration and fast transitions between the carbonation and calcination stages. Our study demonstrates that the sorbent derived from dolomite has a greater capture capacity as compared to limestone. SEM analysis shows that MgO grains in the decomposed dolomite are resistant to sintering under severe calcination conditions and segregate from CaO acting as a thermally stable support which mitigates the multicyclic loss of CaO conversion. Moreover, full decomposition of dolomite is achieved at significantly lower calcination temperatures as compared to limestone, which would help improving further the industrial competitiveness of the technology.
Febrero, 2015 | DOI: 10.1016/j.apenergy.2014.10.087
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
Structural and chemical reactivity modifications of a cobalt perovskite induced by Sr-substitution. An in situ XAS study
Hueso, JL; Holgado, JP; Pereniguez, R; Gonzalez-DelaCruz, VM; Caballero, AMaterials Chemistry and Physics, 151 (2015) 29-33
Show abstract ▽
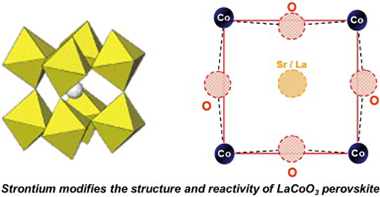
LaCoO3 and La0.5Sr0.5O3O3-delta perovskites have been studied by in situ Co K-edge XAS. Although the partial substitution of La(III) by Sr(II) species induces an important increase in the catalytic oxidation activity and modifies the electronic state of the perovskite, no changes could be detected in the oxidation state of cobalt atoms. So, maintaining the electroneutrality of the perovskite requires the generation of oxygen vacancies in the network. The presence of these vacancies explains that the substituted perovskite is now much more reducible than the original LaCoO3 perovskite. As detected by in situ XAS, after a consecutive reduction and oxidation treatment, the original crystalline structure of the LaCoO3 perovskite is maintained, although in a more disordered state, which is not the case for the Sr doped perovskite. So, the La0.5Sr0.5CoO3-delta perovskite submitted to the same hydrogen reduction treatment produces metallic cobalt, while as determined by in situ XAS spectroscopy the subsequent oxidation treatment yields a Co(III) oxide phase with spinel structure. Surprisingly, no Co(II) species are detected in this new spinel phase.
Febrero, 2015 | DOI: 10.1016/j.matchemphys.2014.11.015
Reactividad de Sólidos
Synthesis of a nanosilica supported CO2 sorbent in a fluidized bed reactor
Soria-Hoyo, C; Valverde, JM; van Ommen, JR; Sanchez-Jimenez, PE; Perez-Maqueda, LA; Sayagues, MJApplied Surface Science, 328 (2015) 548-553
Show abstract ▽
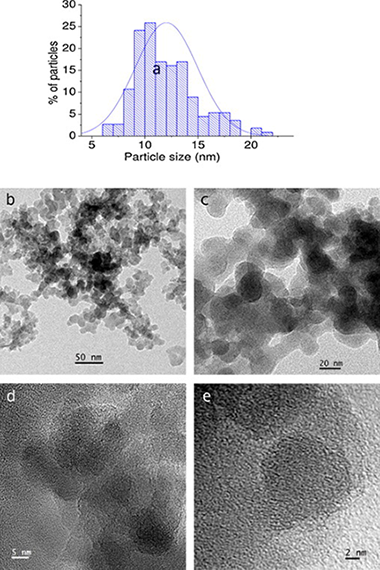
CaO has been deposited on a nanosilica powder matrix by a procedure based on atomic layer deposition (ALD) in a fluidized bed reactor at atmospheric pressure following a potentially scalable process. In previous works ALD in gas fluidized bed has been mostly performed under reduced pressure, which hampers scaling-up the production technology. The material synthesized in the present work is tested as CO2 solid sorbent at calcium looping conditions. Multicyclic thermogravimetric analysis (TGA) shows that the nanosilica support stabilizes the capture capacity of CaO. EDX-STEM analysis illustrates the presence of Ca well distributed on the surface of the SiO2 nanoparticles.
Febrero, 2015 | DOI: 10.1016/j.apsusc.2014.12.106
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
Water splitting performance of Er3+-doped YVO4 prepared from a layered K3V5O14 precursor
Obregon, S; Colon, GChemical Engineering Journal, 262 (2015) 29-33
Show abstract ▽
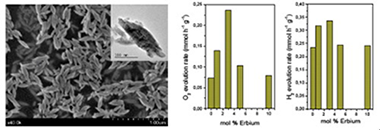
Erbium-doped YVO4 have been synthesized by means of a simple solution method having good photo activities under UV-like excitation for the water splitting half reactions. From the structural and morphological characterization it has been stated that the presence of Er3+ induces the promotion of luminescence. Moreover the incorporation of erbium clearly affects to the morphology YVO4 leading to 200 nm size well-defined spindle-like particles. The improved photocatalytic performance might be associated to a better electron–hole separation mechanism, probably due to the slight increase of band-gap value. The obtained photoactivities for H2 and O2 evolution reactions make this material a promising candidate for water splitting reactions.
Febrero, 2015 | DOI: 10.1016/j.cej.2014.09.073
- ‹ anterior
- 258 of 420
- siguiente ›














