Artículos SCI
2011
2011
Nanotecnología en Superficies y Plasma
Nitrogen plasma functionalization of low density polyethylene
Lopez-Santos, C; Yubero, F; Cotrino, J; Gonzalez-Elipe, ARSurface and Coatings Technology, 205 (2011) 3356-3365
Show abstract ▽
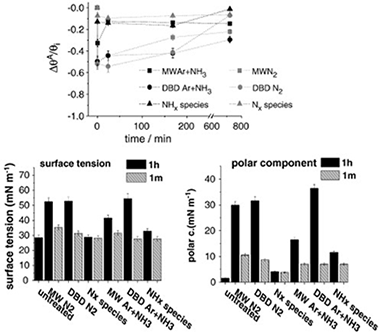
Low density polyethylene (LDPE) films have been treated with different nitrogen containing plasmas with the purpose of incorporating nitrogen functional groups on its surface and analyzing the changes experienced in their surface tension. Effects of a dielectric barrier discharge (DBD) at atmospheric pressure and a microwave discharge (MW) at reduced pressure are compared with those obtained by using an atom source supplied with N2 and mixtures Ar+NH3 as plasma gas. X-ray photoelectron spectroscopy (XPS) analysis has provided information about the chemical surface changes whereas the surface topography of the treated samples has been examined by atomic force microscopy (AFM). Non-destructive depth profiles of oxygen and carbon have been obtained for the treated and one month aged samples by means of the non-destructive Tougaard's method of XPS background analysis. Generally, an oxygen enrichment of the deeper region of treated LDPE surfaces has been observed. Chemical derivatization of the treated samples has shown that a DBD plasma with a mixture of Ar+NH3 was the most efficient treatment for nitrogen and amine group functionalization. It is argued that the high concentration of NH* species in this plasma is the most important factor in enhancing the nitrogen functionalization of this polymer. It has been also found that the observed increase in hydrophilicity and surface tension cannot be attributed to the anchored nitrogen functional groups formed on plasma treated LDPE. Differences in the plasma activation behaviour of LDPE and that of other polymers subjected to similar treatments are stressed.
Febrero, 2011 | DOI: 10.1016/j.surfcoat.2010.11.038
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
Modifying the Size of Nickel Metallic Particles by H2/CO Treatment in Ni/ZrO2 Methane Dry Reforming Catalysts
Gonzalez-Delacruz, VM; Pereñiguez, R; Ternero, F; Holgado, JP; Caballero, AACS Catalysis, 1 (2011) 82-88
Show abstract ▽
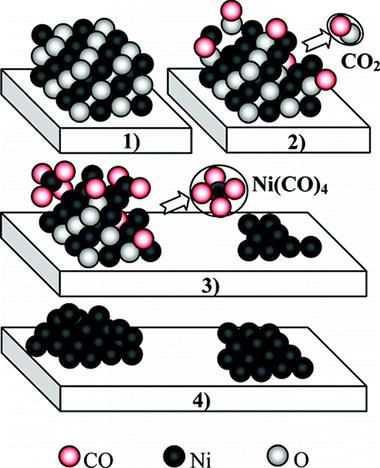
The effect of a reduction process with CO or H-2 on the Size of nickel particles in Ni/ZrO2 dry methane reforming catalysts have been studied by means of in situ X-ray Spectroscopy (XAS) and Diffuse Reflectance FTIR Spectroscopy (DRIFTS). Our results clearly indicate that a high temperature treatment with CO increases the dispersion of the nickel metallic phase. XAS results have shown a lower coordination number of Ni in the sample treated with CO than that reduced with H-2. From the DRIFTS results, it can he established that, under the CO treatment, the formation of Ni(CO)(4) complexes corrodes the nickel particles, decreasing their size. The formation of these gas molecules occurs without measurable losses of nickel from the catalyst which maintains the same nickel content after the hydrogen or the CO treatment at high temperature:Therefore, this airborne nickel compound, by colliding with the zirconia surface, must deposit the nickel metal metal atoms around onto the support. This behavior is evidence of an important interaction b etween nickel and zirconia surface as unlike other supports there is no losses of nickel during the dispersion process on zirconia. Although different effects of CO on nickel catalysts have been previously described, we have found for the first time several experimental evidences demonstrating the whole redispersion phenomenon.
Febrero, 2011 | DOI: 10.1021/cs100116m
Nanotecnología en Superficies y Plasma
Transparent Nanometric Organic Luminescent Films as UV-Active Components in Photonic Structures
Aparicio, FJ; Holgado, M; Borras, A; Blaszczyk-Lezak, I; Griol, A; Barrios, CA; Casquel, R; Sanza, FJ; Sohlstrom, H; Antelius, M; Gonzalez-Elipe, AR; Barranco, AAdvanced Materials, 23 (2011) 761-765
Show abstract ▽
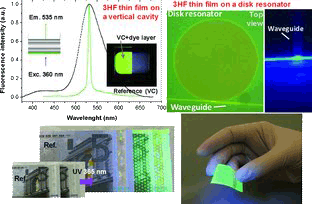
A new kind of visible-blind organic thin-film material, consisting of a polymeric matrix with a high concentration of embedded 3-hydroxyflavone (3HF) dye molecules, that absorbs UV light and emits green light is presented. The thin films can be grown on sensitive substrates, including flexible polymers and paper. Their suitability as photonic active components photonic devices is demonstrated.
Febrero, 2011 | DOI: 10.1002/adma.201003088
Nanotecnología en Superficies y Plasma
Selective Dichroic Patterning by Nanosecond Laser Treatment of Ag Nanostripes
Sanchez-Valencia, JR; Toudert, J; Borras, A; Barranco, A; Lahoz, R; de la Fuente, GF; Frutos, F; Gonzalez-Elipe, ARAdvanced Materials, 23 (2011) 848-853
Show abstract ▽
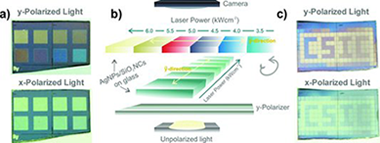
A simple route for the fabrication of dichroic optical structures based on Ag nanoparticles deposited onto SiO2 nanocolumns is presented. The strict control of the optical response is achieved after infrared laser treatment of the supported nanoparticles with a commercial nanosecond pulsed laser. Preliminary examples of the utilization of the laser-treated AgNPs/SiO2 nanocolumn system for optical recoding and encryption are shown.
Febrero, 2011 | DOI: 10.1002/adma.201003933
Química de Superficies y Catálisis
Thermal analysis of monument patina containing hydrated calcium oxalates
Perez-Rodriguez, JL; Duran, A; Centeno, MA; Martinez-Blanes, JM; Robador, MDThermochimica Acta, 512 (2011) 5-12
Show abstract ▽
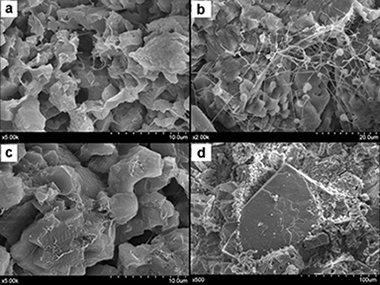
This work describes the thermal transformation of patina samples formed on the surface of dolomitic rocks used to build the Romanesque Torme's Church (Burgos, Spain). Analyses were performed using a combination of high-temperature XRD, simultaneous TG/DTA and gas mass spectrometry. The XRD analysis revealed the presence of hydrated calcium oxalates. The following three steps were proposed for the thermal transformation of the raw material: dehydration of weddellite/whewellite to form calcium oxalate, transformation of calcium oxalate to calcium carbonate, and formation of calcium oxide produced via decomposition of the calcite. DTA/TG and mass spectrometry analyses confirmed this mechanism. In addition, a high proportion of organic compounds was detected and was possibly formed via degradation of products applied for the building's conservation by the action of microorganisms attack. Mass spectrometry analysis revealed CO (and CO2) gas evolved during the transformation of CaC2O4 to CaCO3. The CO2 gas also appears at 765 °C due to the decomposition of calcium carbonate, and it appears over a large range of temperatures due to the decomposition of organic compounds. The TG analyses performed in a CO2 atmosphere were used to determine the percentages of Ca and Mg contained in dolomite, and the calcium carbonate formed by oxalate decomposition. DRIFTS and mass spectrometry results revealed the presence of several aliphatic and/or aromatic compounds containing CO groups.
Enero, 2011 | DOI: 10.1016/j.tca.2010.08.015
- ‹ anterior
- 362 of 422
- siguiente ›














