Artículos SCI
2011
2011
Intercalation and dynamics of hydrated Fe2+ in the vermiculites from Santa Olalla and Ojén
Anton Lerf, Friedrich E. Wagner, Juan Poyato and José Luis Pérez-RodríguezJournal of Solid State Electrochemistry, 15 (2011) 223-229
Show abstract ▽
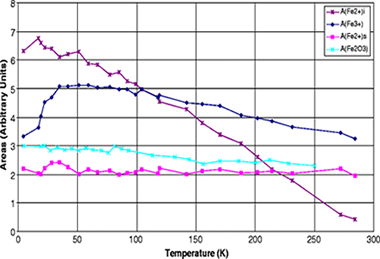
Although the intercalation of Fe3+ into layered phyllosicilicates-especially into smectites-attracted much attention in the past two decades, the information about Fe2+ loaded phyllosilicates is sparse. Here we present an investigation of the Fe2+ exchanged vermiculites from Santa Olalla and Ojén (Andalusia, Spain) by means of Mössbauer spectroscopy. The room temperature Mössbauer spectra are very similar to those of the starting compounds (Na forms) except for a decrease of the contribution of structural Fe3+ and a concomitant increase of the contribution of Fe2+ sites, indicating an internal redox process. The extent of this redox reaction is different for the two vermiculites. Thus, the intercalated Fe2+ acts as an electron mediator from the external medium to the structural Fe3+ ions. A new component attributable to intercalated Fe2+ is practically invisible in the room temperature Mössbauer spectra, but increases strongly and continuously during cooling to 4.2 K, where it is the dominant feature of the Mössbauer patterns. At 4.2 K, its quadruple splitting amounts to 3.31 mm/s, which is in excellent agreement with the quadrupole slitting of Fe2+ coordinated to six water molecules in a highly symmetric octahedral arrangement. The strong decrease of the Mössbauer-Lamb factor of this component with increasing temperature indicates a weak bonding of the Fe 2+ in the interlayer space.
Febrero, 2011 | DOI: 10.1007/s10008-010-1171-0
Nanotecnología en Superficies y Plasma
Nitrogen plasma functionalization of low density polyethylene
Lopez-Santos, C; Yubero, F; Cotrino, J; Gonzalez-Elipe, ARSurface and Coatings Technology, 205 (2011) 3356-3365
Show abstract ▽
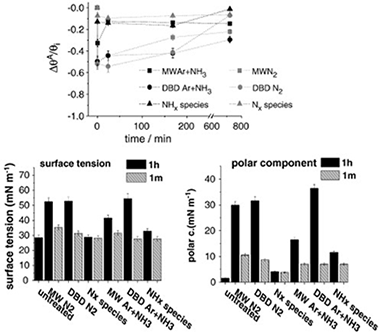
Low density polyethylene (LDPE) films have been treated with different nitrogen containing plasmas with the purpose of incorporating nitrogen functional groups on its surface and analyzing the changes experienced in their surface tension. Effects of a dielectric barrier discharge (DBD) at atmospheric pressure and a microwave discharge (MW) at reduced pressure are compared with those obtained by using an atom source supplied with N2 and mixtures Ar+NH3 as plasma gas. X-ray photoelectron spectroscopy (XPS) analysis has provided information about the chemical surface changes whereas the surface topography of the treated samples has been examined by atomic force microscopy (AFM). Non-destructive depth profiles of oxygen and carbon have been obtained for the treated and one month aged samples by means of the non-destructive Tougaard's method of XPS background analysis. Generally, an oxygen enrichment of the deeper region of treated LDPE surfaces has been observed. Chemical derivatization of the treated samples has shown that a DBD plasma with a mixture of Ar+NH3 was the most efficient treatment for nitrogen and amine group functionalization. It is argued that the high concentration of NH* species in this plasma is the most important factor in enhancing the nitrogen functionalization of this polymer. It has been also found that the observed increase in hydrophilicity and surface tension cannot be attributed to the anchored nitrogen functional groups formed on plasma treated LDPE. Differences in the plasma activation behaviour of LDPE and that of other polymers subjected to similar treatments are stressed.
Febrero, 2011 | DOI: 10.1016/j.surfcoat.2010.11.038
Study of ground and unground leached vermiculite II. Thermal behaviour of ground acid-treated vermiculite
Perez-Rodriguez, JL; Maqueda, C; Murafa, N; Subrt, J; Balek, V; Pulisova, P; Lancok, AApplied Clay Science, 51 (2011) 274-282
Show abstract ▽
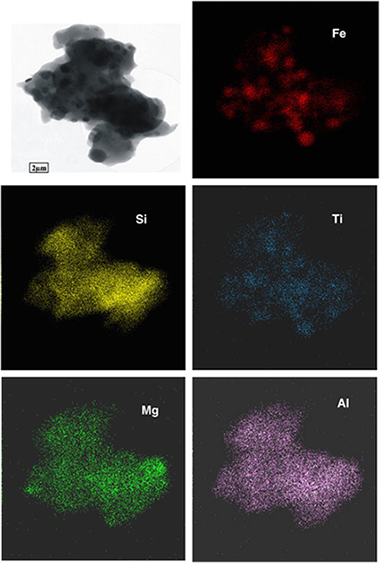
In this study, we examined the annealing effect on the material obtained after acid treatment of ground vermiculite which constituted amorphous silica and β-FeOOH. The XRD patterns of the starting sample measured at temperatures from 30 to 1200°C showed that the crystalline phase was present until ~300°C; whereas the sample heated between 300 and 800°C was practically amorphous. This is in agreement with previous observations that β-FeOOH decomposes to amorphous or poorly crystalline phase, β-Fe2O3, and transforms only slowly to crystalline α-Fe2O3. At 850°C the sample showed the first signs of a crystalline phase which was fully developed at 1050°C. The XRD, HRTEM and Mössbauer spectroscopy showed, after heating at 1050°C, the presence of crystalline phase, consisting of quartz, cristobalite, α-Fe2O3 and ε-Fe2O3. This effect showed in fact that well crystallized iron oxide nanoparticles embedded into the silica matrix are usually formed at relative high temperatures (~1000°C), which is in contrast to silica-free material. Element mapping of one particle of the composite obtained by annealing the sample at the highest temperature showed well-separated Fe2O3 and SiO2 particles in a composite material. Impurities of Al and Mg (from the original vermiculite) accompanied the silica components and TiO2 associated with Fe2O3 grains was also detected.
Febrero, 2011 | DOI: 10.1016/j.clay.2010.11.031
Nanotecnología en Superficies y Plasma
Lateral and in-depth distribution of functional groups on diamond-like carbon after oxygen plasma treatments
Lopez-Santos, C; Yubero, F; Cotrino, J; Gonzalez-Elipe, ARDiamond and Related Materials, 20 (2011) 49-56
Show abstract ▽
A diamond like carbon material has been exposed to a low pressure microwave and atmospheric pressure plasma of oxygen to enhance its hydrophilicity and surface energy. For comparison, data are also reported after activation with a beam of neutral atoms of oxygen. The surface incorporation of oxygenated functional groups and the determination of the in-depth distribution of this element have been analysed by means of the X ray photoemission spectroscopy (XPS). Atomic force microscopy (AFM) has been used to get information of the surface topography and, by recording friction maps of the surface, the lateral distribution of oxygenated functional groups formed after the different activation treatments. Differences in surface composition, topography and in-depth and lateral distribution of oxygen have been correlated with the intrinsic characteristics of the activation plasma processes.
Febrero, 2011 | DOI: 10.1016/j.diamond.2010.11.024
Materiales de Diseño para la Energía y Medioambiente
Influence of OH− concentration on the illitization of kaolinite at high pressure
M. Mantovani, A. Escudero, A.I. Becerro,Applied Clay Science, 51 (2011) 220-225
Show abstract ▽
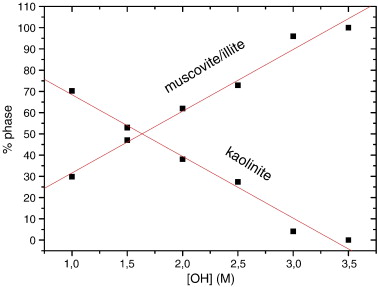
The products of hydrothermal reactions of kaolinite at 300 °C and 1000 bars were studied in KOH solutions covering an OH− concentration, [OH−], of 1 M to 3.5 M. XRD patterns indicated a notable influence of the [OH−] on the reaction. At [OH] ≥ 3 M, the only stable phase was muscovite/illite. The content of muscovite/illite was calculated from the analysis of the diagnostic 060 reflections of kaolinite and muscovite/illite. The results showed a linear dependence of kaolinite and muscovite/illite contents with [OH−]. 27Al MAS NMR spectroscopy revealed the formation of small nuclei of K-F zeolite at high [OH−]. Finally, modelling of the 29Si MAS NMR spectra indicated that the Si/Al ratio of the muscovite/illite formed was very close to that of muscovite, at least in the mineral formed at low [OH−]. In good agreement with the XRD data, the quantification of the reaction products by 29Si MAS NMR indicated a linear decrease of the kaolinite content with increasing OH− concentration.
Febrero, 2011 | DOI: 10.1016/j.clay.2010.11.021
- ‹ anterior
- 361 of 422
- siguiente ›














