Artículos SCI
2010
2010
Reactividad de Sólidos - Fotocatálisis Heterogénea: Aplicaciones
The Multistep Nature of the Kaolinite Dehydroxylation: Kinetics and Mechanism
Ortega, A; Macias, M; Gotor, FJJournal of the American Ceramic Society, 93 (2010) 197-203
Show abstract ▽

The thermal dehydroxylation of kaolinite has been reexamined using small sample weights, a homogeneous particle size distribution and high-vacuum conditions in order to reduce the influences of heat and mass-transfer phenomena. The controlled rate thermal analysis (CRTA) technique, which was specially developed to minimize the pressure and temperature gradients through the sample, was employed to carry out meaningful kinetic experiments. Two advanced isoconversional methods, the Vyazovkin and the Galwey methods, were used complementarily to determine the dependence of the activation energy on the degree of conversion. For this purpose, the Vyazovkin method has been adapted to CRTA experiments. It was demonstrated that there are at least two different stages, revealing the multistep nature of this reaction. The activation energy for the first step, which is assigned to nucleation and the growth of nuclei, decreases from 100 to 75 kJ/mol. The second stage corresponds to a diffusion process and the activation energy rises to 120 kJ/mol because of the metakaolinite formation, which closes the interlamellar channels and leaves isolated patches of kaolinite from which the water escapes with difficulty.
Enero, 2010 | DOI: 10.1111/j.1551-2916.2009.03328.x
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
Chemical and electronic characterization of cobalt in a lanthanum perovskite. Effects of strontium substitution
Hueso, JL; Holgado, JP; Pereniguez, R; Mun, S; Salmeron, M; Caballero, AJournal of Solid State Chemistry, 183 (2010) 27-32
Show abstract ▽
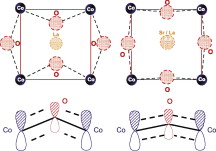
Two different cobaltites, LaCoO3 and La0.5Sr0.5CoO3-delta, have been prepared and characterized by means of high energy Co K-edge and low energy O K-edge X-ray absorption spectroscopy (XAS). Even though half of the La(III) is substituted by Sr(II), little or no changes call be detected in the formal oxidation state of cobalt atoms. The presence of strontium cations induces two main effects in the chemical and electronic state of the perovskite. The charge balance with Sr(II) species is reached by the formation of oxygen vacancies throughout the network, which explains the well-known increase in the reactivity of this Substituted perovskite. O K-edge XAS experiments show that the Sr(II) species induce the transitions of d electrons of cobalt cations from low to high Spill Configuration. We propose that this change in Spill Multiplicity is induced by two cooperative effects: the oxygen vacancies. creating five coordinated cobalt atoms, and the bigger size of Sr(II) cations, aligning the Co-O-Co atoms, and favoring the overlapping of pi-symmetry cobalt and oxygen orbitals, reducing the splitting energy of e(g) and t(2g) levels.
Enero, 2010 | DOI: 10.1016/j.jssc.2009.10.008
Química de Superficies y Catálisis
Supported nickel catalysts with a controlled molecular architecture for the catalytic reformation of methane
Hufschmidt, D; Bobadilla, LF; Romero-Sarria, F; Centeno, MA; Odriozola, JA; Montes, M; Falabella, ECatalysis Today, 149 (2010) 394-400
Show abstract ▽

In this work a lanthanum and nickel catalyst having perovskite structure, grown on a gamma-alumina carrier, is being presented. The structure of the catalyst was confirmed by XRD. The behaviour of this material under the conditions of steam reforming has been studied and the influence of the temperature, the space velocity and the steam/carbon ratio on the conversion of methane and the product distribution in the process was determined. In all cases at higher temperatures conversions of more than 90% and high selectivities were achieved. The experiments to determine the stability of the catalyst demonstrated that no deactivation in experimental runs of more than 17h occurred. Additionally a study of the catalyst after the reaction showed that only lowly structured carbonaceous species were formed on the catalyst surface, which is not expected to inhibit strongly the initial catalytic activity.
Enero, 2010 | DOI: 10.1016/j.cattod.2009.06.002
Nanotecnología en Superficies y Plasma - Materiales Ópticos Multifuncionales
Conformal Growth of Organic Luminescent Planar Defects within Artificial Opals
Aparicio, FJ; Lozano, G; Blaszczyk-Lezak, I; Barranco, A; Miguez, HChemistry of Materials, 22 (2010) 379-385
Show abstract ▽
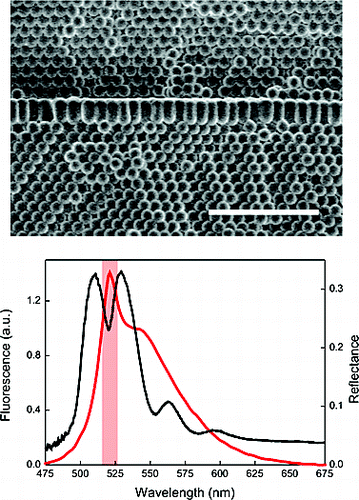
Herein, we present the result of combining, for the first time, the techniques of colloidal self-assembly and plasma-enhanced chemical vapor deposition to create a novel, high-quality, purely Organic active photonic crystal structure of controlled optical properties. We show a fast. reliable, and accurate procedure to introduce two-dimensional luminescent organic defect layers within artificial polystyrene opals via a versatile room-temperature remote plasma deposition process. This method is gentle enough to allow highly coil formal growth on polystyrene microspheres without altering their morphology or the ordered arrangement that they form. The luminescent organic layer behaves both as all optical dopant, causing the opening of transmission windows within the forbidden frequency interval of the lattice, and as an optically active material, whose emission call be tailored by the photonic environment.
Enero, 2010 | DOI: 10.1021/cm902819x
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
Synthesis and characterization of a LaNiO3 perovskite as precursor for methane reforming reactions catalysts
Pereñiguez, R; Gonzalez-DelaCruz, VM; Holgado, JP; Caballero, AApplied Catalysis B-Environmental, 93 (2010) 346-353
Show abstract ▽
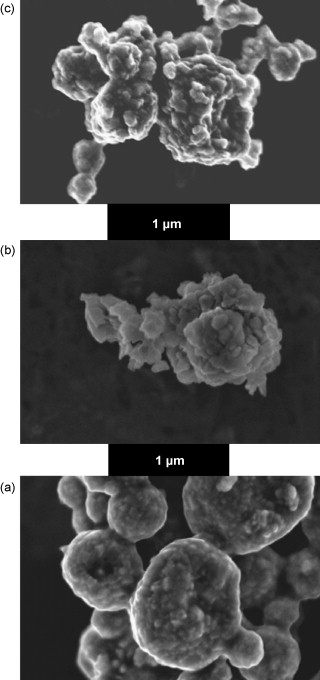
The objective of the present work has been the study of the physicochemical and catalytic properties of a Ni/La2O3 catalyst obtained by reduction of a lanthanum nickelite, LaNiO3, with perovskite structure. The perovskite, obtained by means of a spray pyrolysis method, provides a Ni/La2O3 system active in different methane reforming reactions. The catalyst was characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray absorption spectroscopy (XAS), X-Ray photoemission spectroscopy (XPS), temperature-programmed reduction and oxidation (TPR, TPO) and catalytic activity tests. Although not evidenced by XRD data, XAS and TPR measurements show the presence of an amorphous NiO phase in the original sample, together with the crystalline LaNiO3 phase. Upon reoxidation treatment of the reduced Ni/La2O3 catalyst, the LaNiO3 structure is partly recovered which provides a convenient way to regenerate a waste catalyst (reoxidation and new reduction in hydrogen). The catalyst is active in several reactions of methane with oxygen, water and CO2, showing a remarkable stability specially under dry reforming of methane (DRM) reaction conditions. This quite great catalytic performance has been explained by the high resistance of the nickel particles to be oxidized, as detected by in situ XAS. In the presence of water, as in steam reforming of methane (SRM) reaction conditions, these metallic particles are gradually oxidized, which explains the linear decreasing of the catalytic performance observed for the SRM reaction.
Enero, 2010 | DOI: 10.1016/j.apcatb.2009.09.040
- ‹ anterior
- 386 of 422
- siguiente ›














