Artículos SCI
2011
2011
Natural earth pigments from Roman and Arabic wall paintings revealed by spectroscopic techniques
Garofano, I., Duran, A., Perez-Rodriguez, J.L., Robador, M.D.Spectroscopy Letters, 44 (2011) 560-565 DOI: 10.1080/00387010.2011.610655
Abstract
Full identification of pigments used in wall paintings by Romans and Arabs that were recently discovered was achieved by the combined application of several spectroscopy methods. Identification of pigments was provided by the use of micro-Raman and FT-IR spectroscopy, while UV-Visible spectroscopy and chromatic studies permitted the authors to identify slight variations of hue attributed to mixtures of pigments. Natural earths and minerals were detected as the main pigments employed by both civilizations, although some differences were found between them. Red ochre, vermilion, yellow ochre, Egyptian blue, green earth, calcite, carbon, and possibly ivory blacks were identified in the Roman paintings. Only hematite and calcite were observed in the Arabic fragments.
Junio, 2011 | DOI: 10.1080/00387010.2011.610655
Materiales de Diseño para la Energía y Medioambiente
Electrical properties of biomorphic SiC ceramics and SiC/Si composites fabricated from medium density fiberboard
T.S. Orlova, V.V. Popov, J. Quispe Cancapa, D. Hernández Maldonado, E. Enrique Magarino, F.M. Varela Feria, A. Ramírez de Arellano, J. Martínez FernándezJournal of the European Ceramic Society, 31 (2011) 1317-1323 DOI: 10.1016/j.jeurceramsoc.2010.06.015

Abstract
A study has been made of the dependences of the electrical resistivity and the Hall coefficient on the temperature in the range 1.8–1300 K and on magnetic fields of up to 28 kOe for the biomorphic SiC/Si (MDF-SiC/Si) composite and biomorphic porous SiC (MDF-SiC) based upon artificial cellulosic precursor (MDF – medium density fiberboards). It has been shown that electric transport in MDF-SiC is effected by carriers of n-type with a high concentration of ∼1020 cm−3 and a low mobility of ∼0.4 cm2 V−1 s−1. The specific features in the conductivity of MDF-SiC are explained by quantum effects arising in disordered systems and requiring quantum corrections to conductivity. The TEM studies confirmed the presence of disordering structural features (nanocrystalline regions) in MDF-SiC. The conductivity of MDF-SiC/Si composite originates primarily from Si component in the temperature range 1.8–500 K and since ∼500 to 600 K the contribution of MDF-SiC matrix becomes dominant.
Junio, 2011 | DOI: 10.1016/j.jeurceramsoc.2010.06.015
Materiales de Diseño para la Energía y Medioambiente
Compressive strength degradation in ZrB2-based ultra-high temperature ceramic composites
Ramirez-Rico, J; Bautista, MA; Martinez-Fernandez, J; Singh, MJournal of the European Ceramic Society, 31 (2011) 1345-1352 DOI: 10.1016/j.jeurceramsoc.2010.05.020
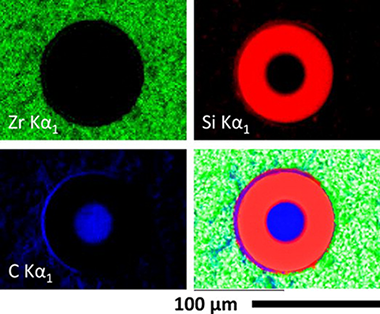
Abstract
The high temperature compressive strength behavior of zirconium diboride (ZrB2)-silicon carbide (SiC) particulate composites containing either carbon powder or SCS-9a silicon carbide fibers was evaluated in air. Constant strain rate compression tests have been performed on these materials at room temperature, 1400, and 1550°C. The degradation of the mechanical properties as a result of atmospheric air exposure at high temperatures has also been studied as a function of exposure time. The ZrB2-SiC material shows excellent strength of 3.1±0.2GPa at room temperature and 0.9±0.1GPa at 1400°C when external defects are eliminated by surface finishing. The presence of C is detrimental to the compressive strength of the ZrB2-SiC-C material, as carbon burns out at high temperatures in air. As-fabricated SCS-9a SiC fiber reinforced ZrB2-SiC composites contain significant matrix microcracking due to residual thermal stresses, and show poor mechanical properties and oxidation resistance. After exposure to air at high temperatures an external SiO2 layer is formed, beneath which ZrB2 oxidizes to ZrO2. A significant reduction in room temperature strength occurs after 16-24h of exposure to air at 1400°C for the ZrB2-SiC material, while for the ZrB2-SiC-C composition this reduction is observed after less than 16h. The thickness of the oxide layer was measured as a function of exposure time and temperatures and the details of oxidation process has been discussed.
Junio, 2011 | DOI: 10.1016/j.jeurceramsoc.2010.05.020
Materiales para Bioingeniería y Regeneración Tisular
Hydroxyapatite Synthesis on Mesoporous Silica: A High Resolution Electron Microscopy Study
D.R. Acosta, A. Díaz-Cuenca, A.Acta Microscopica, 20 (2011) 29-35 DOI:
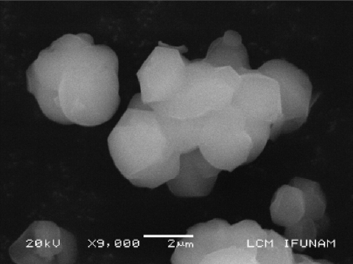
Abstract
En este trabajo se presentan resultados de la síntesis de hidroxiapatita (HA) en sílice mesoporosa SBA-15. Se ha hecho un estudio de la síntesis de ambos materiales y un seguimiento del efecto del doble tratamiento térmico posterior a la síntesis. Las muestras se sometieron a distintas temperaturas de tratamiento hidrotermal entre 353 y 393 K con incrementos de 10 K durante 24 horas. En cada caso y una vez filtrado y seco el material se volvió a tratar con una calcinación a 773 K durante 10 hs. Se presentan los resultados del estudio del material compuesto SBA-15-HA por microscopia electrónica de transmisión convencional y avanzada ( STEM, Contraste Z, HREM) . El crecimiento de HA en los túneles de la matriz de sílice mesoporosa y el nivel de ocupación de los mismos aumenta con la temperatura del primer tratamiento hidrotermal y también del segundo tratamiento que favorece el sinterizado dentro de los túneles.
Mayo, 2011 | DOI:
Materiales de Diseño para la Energía y Medioambiente
Stability of Rare-Earth Disilicates: Ionic Radius Effect
Galunin, E; Alba, MD; Vidal, MJournal of the American Ceramic Society, 94 (2011) 1568–1574 DOI: 10.1111/j.1551-2916.2010.04272.x
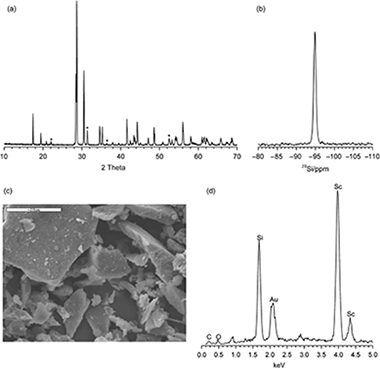
Abstract
Rare-earth (RE) disilicates, of general formula RE2Si 2O7, are one of the products of the chemical reaction between RE (elements), which are actinide simulators, and the silicates used in the engineered barrier systems of deep geological repositories (DGPs). The aim of this paper is to establish the stability range of the disilicate phase as function of the nature of the RE (RE=Sc, Lu, or Y) and examine whether this phase would permit RE leaching under experimental conditions simulating those of the DGP. The β-polymorphs of the RE disilicates were synthesized by the sol-gel method and subsequently submitted to a pHstat leaching test. The rates of RE and Si leaching were measured and the transformation of the crystalline and amorphous phases was examined by X-ray powder diffraction and nuclear magnetic resonance techniques. The results indicate that the disilicate phases were stable within a wide range of pH, their stability being related to the hydrated ratio of the RE. Disilicate stability increased with the ionic radius of the RE. As a result, the Sc disilicate was stable throughout the pH range tested, whereas Y and Lu disilicate leaching was only observed at pH<4. Thus, it was confirmed that the formation of the disilicate phases could contribute to the confinement of radioactive wastes in engineered barriers.
Mayo, 2011 | DOI: 10.1111/j.1551-2916.2010.04272.x
Materiales para Bioingeniería y Regeneración Tisular
Determination of Pore Size Distribution at the Cell-Hydrogel Interface
Leal-Egana, A., Dietrich-Braumann, U., Diaz-Cuenca, A., Nowicki, M., Bader, A.Journal of Nanobiotechnology, 9 (2011) Page 24 DOI: 10.1186/1477-3155-9-24
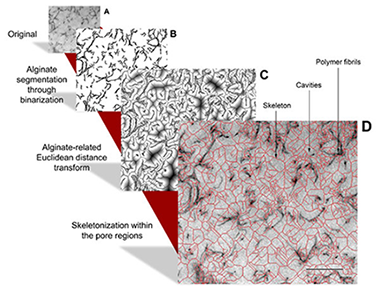
Abstract
Analyses of the pore size distribution in 3D matrices such as the cell-hydrogel interface are very useful when studying changes and modifications produced as a result of cellular growth and proliferation within the matrix, as pore size distribution plays an important role in the signaling and microenvironment stimuli imparted to the cells. However, the majority of the methods for the assessment of the porosity in biomaterials are not suitable to give quantitative information about the textural properties of these nano-interfaces.
Mayo, 2011 | DOI: 10.1186/1477-3155-9-24
Materiales de Diseño para la Energía y Medioambiente
Structure and support induced structure disruption of soft nanoparticles obtained from hydroxylated fatty acids
Heredia-Guerrero, JA; San-Miguel, MA; Luna, M; Dominguez, E; Heredia, A; Benitez, JJSoft Matter, 7 (2011) 4357-4363 DOI: 10.1039/c0sm01545h
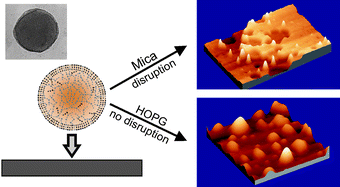
Abstract
Soft and spherical nanoparticles, named as cutinsomes, have been prepared from concentrated 9(10),16-dihydroxypalmitic acid (diHPA) in aqueous solution. After isolation, cutinsomes have been chemically and structurally characterized by ATR-FTIR, TEM and dynamic atomic force microscopy (dynamic AFM). The nanoparticle can be described as a lipidic, liquid-like and mostly esterified core surrounded by a polar shell of carboxylate/carboxylic acid molecules. Molecular dynamic (MD) simulations have been used to support this model. The structural stability of soft cutinsomes has been tested by deposition on both non-polar (HOPG) and polar (mica) flat substrates. It has been found that the magnitude of the interaction between the polar shell of cutinsomes and the support determines their structure conservation or its spreading or rupture and spill out of the liquid-like content. The structural consistence of these nanoparticles as a function of the polarity of substrate is of interest in elucidating the formation mechanism of cutin, the most abundant biopolyester in nature and a very interesting biomaterial to be mimetized.
Mayo, 2011 | DOI: 10.1039/c0sm01545h
Materiales de Diseño para la Energía y Medioambiente
Interaction of Eu-isotopes with saponite as a component of the engineered barrier
María D. Alba, Miguel A. Castro, P. Chaín, Santiago Hurtado, M. Mar Orta, M. Carolina Pazos and María VillaApplied Clay Science, 52 (2011) 253-257 DOI: 10.1016/j.clay.2011.02.027

Abstract
Bentonite is accepted as the best clay material in the engineered barrier of deep geological repositories (DGRs) for radioactive waste disposal. In recent years, the interactions between a wide range of rare-earth (REE) cations and smectites have been studied. A combined study of stable europium and radioactive isotopes is reported here. Saponite was subjected to hydrothermal reactions with stable and radioactive (152Eu) europium ions under subcritical conditions. The structural changes of saponite were evaluated by XRD and SEM. The effect of temperature and reaction time on the changes was quantified by measuring 152Eu through gamma spectrometry. The reaction between europium and saponite was a first-order reaction. The presence of Eu in the precipitate in an amount much higher than the cation exchange capacity of saponite confirmed participation of chemical reactions or surface adsorption in the europium immobilization, even at temperatures as low as 150 °C. The reaction rate constant indicated that an 8- to 9-month period was needed for the completion, without significant changes, of the europium/saponite chemical reaction under the subcritical conditions of 200 °C and 350 °C.
Mayo, 2011 | DOI: 10.1016/j.clay.2011.02.027
Materiales Ópticos Multifuncionales
Interplay of Resonant Cavity Modes with Localized Surface Plasmons: Optical Absorption Properties of Bragg Stacks Integrating Gold Nanoparticles
Olalla Sánchez-Sobrado, Gabriel Lozano, Mauricio E. Calvo, Ana Sánchez-Iglesias, Luis M. Liz-Marzán, Hernán MíguezAdvanced Materials, 23 (2011) 2108-2112 DOI: 10.1002/adma.201004401

Abstract
A procedure to prepare porous photonic crystal resonators containing gold nanoparticles is reported. The optical absorption of the ensemble, resulting from the excitation of the localized surface plasmon of the metallic beads, is finely tuned by a gradual shift of the cavity mode. This is achieved by infiltration of the void network with different guest compounds.
Mayo, 2011 | DOI: 10.1002/adma.201004401
Nanotecnología en Superficies y Plasma
Supported plasma-made 1D heterostructures: Perspectives and applications
Borras, A; Macias-Montero, M; Romero-Gomez, P; Gonzalez-Elipe, ARJournal of Physics D: Applied Physics, 44 (2011) 174016 DOI: 10.1088/0022-3727/44/17/174016
Abstract
Plasma-related methods have been widely used in the fabrication of carbon nanotubes and nanofibres (NFs) and semiconducting inorganic nanowires (NWs). A natural progression of the research in the field of 1D nanostructures is the synthesis of multicomponent NWs and NFs. In this paper we review the state of the art of the fabrication by plasma methods of 1D heterostructures including applications and perspectives. Furthermore, recent developments on the use of metal seeds (Ag, Au, Pt) to obtain metal@oxide nanostructures are also extensively described. Results are shown for various metal substrates, either metal foils or supported nanoparticles/thin films of the metal where the effects of the size, surface coverage, percolation degree and thickness of the metal seeds have been systematically evaluated. The possibilities of the process are illustrated by the preparation of nanostructured films and supported NFs of different metal@oxides (Ag, Au and SiO2, TiO2, ZnO). Particularly, in the case of silver, the application of an oxygen plasma treatment prior to the deposition of the oxide was critical for efficiently controlling the growth of the 1D heterostructures. A phenomenological model is proposed to account for the thin-film nanostructuring and fibre formation by considering basic phenomena such as stress relaxation, inhomogeneities in the plasma sheath electrical field and the local disturbance of the oxide growth.
Mayo, 2011 | DOI: 10.1088/0022-3727/44/17/174016
Reactividad de Sólidos
Constant rate thermal analysis for thermal stability studies of polymers
Sanchez-Jimenez, PE; Perez-Maqueda, LA; Perejon, A; Criado, JMPolymer Degradation and Stability, 96 (2011) 974-981 DOI: 10.1016/j.polymdegradstab.2011.01.027
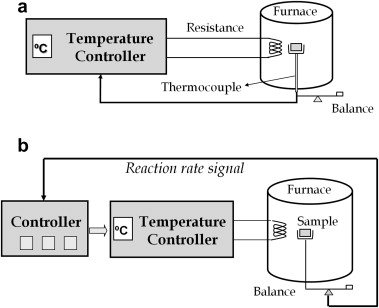
Abstract
This paper explores the relationship between the shapes of temperature-time curves obtained from experimental data recorded by means of constant rate thermal analysis (CRTA) and the kinetic model followed by the thermal degradation reaction. A detailed shape analysis of CRTA curves has been performed as a function of the most common kinetic models. The analysis has been validated with simulated data, and with experimental data recorded from the thermal degradation of polytetrafluoroethylene (PTFE), poly(1,4-butylene terephthalate) (PBT), polyethylene (PE) and poly(vinyl chloride) (PVC). The resulting temperature-time profiles indicate that the studied polymers decompose through phase boundary, random scission, diffusion and nucleation mechanisms respectively. The results here presented demonstrate that the strong dependence of the temperature-time profile on the reaction mechanism would allow the real kinetic model obeyed by a reaction to be discerned from a single CRTA curve.
Mayo, 2011 | DOI: 10.1016/j.polymdegradstab.2011.01.027
Materiales Ópticos Multifuncionales
Analysis of artificial opals by scanning near field optical microscopy
Barrio, J; Lozano, G; Lamela, J; Lifante, G; Dorado, LA; Depine, RA; Jaque, F; Miguez, HJ. Appl. Phys., 109 (2011) 083514(5 pages) DOI: 10.1063/1.3573777
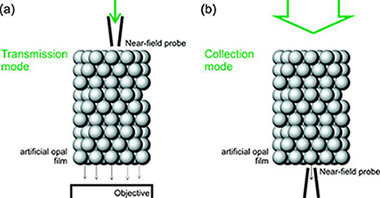
Abstract
Herein we present a detailed analysis of the optical response of artificial opal films realized employing a near-field scanning optical microscope in collection and transmission modes. Near-field patterns measured at the rear surface when a plane wave impinges on the front face are presented with the finding that optical intensity maps present a clear correlation with the periodic arrangement of the outer surface. Calculations based on the vector Korringa-Kohn-Rostoker method reproduce the different profiles experimentally observed as well as the response to the polarization of the incident field. These observations constitute the first experimental confirmation of the collective lattice resonances that give rise to the optical response of these three dimensional periodic structures in the high-energy range.
Abril, 2011 | DOI: 10.1063/1.3573777
Materiales de Diseño para la Energía y Medioambiente
Structural, chemical surface and transport modifications of regenerated cellulose dense membranes due to low-dose γ-radiation
Vazquez, MI; Heredia-Guerrero, JA; Galan, P; Benitez, JJ; Benavente, JMaterials Chemistry and Physics, 126 (2011) 734-740 DOI: 10.1016/j.matchemphys.2010.12.051
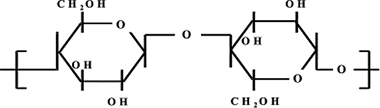
Abstract
Modifications caused in commercial dense regenerated cellulose (RC) flat membranes by low-dose γ-irradiation (average photons energy of 1.23 MeV) are studied. Slight structural, chemical and morphological surface changes due to irradiation in three films with different RC content were determined by ATR-FTIR, XRD, XPS and AFM. Also, the alteration of their mechanical elasticity has been studied. Modification of membrane performance was determined from solute diffusion coefficient and effective membrane fixed charge concentration obtained from NaCl diffusion measurements. Induced structural changes defining new and effective fracture propagation directions are considered to be responsible for the increase of fragility of irradiated RC membranes. The same structural changes are proposed to explain the reduction of the membrane ion permeability through a mechanism involving either ion pathways elongation and/or blocking.
Abril, 2011 | DOI: 10.1016/j.matchemphys.2010.12.051
Materiales de Diseño para la Energía y Medioambiente
Bulk and Adsorbed Monolayer Phase Behavior of Binary Mixtures of Undecanoic Acid and Undecylamine: Catanionic Monolayers
Sun, C; Bojdys, MJ; Clarke, SM; Harper, LD; Jefferson, A; Castro, MA; Medina, SLangmuir, 27 (2011) 3626-3637 DOI: 10.1021/la1048198
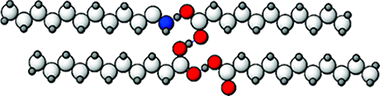
Abstract
Differential scanning calorimetry (DSC) and X-ray powder diffraction (PXRD) have been used to determine the phase behavior of the binary mixtures of undecanoic acid (A) and undecylamine (B) in the bulk. In addition, we report DSC data that indicates very similar behavior for the solid monolayers of these materials adsorbed on the surface of graphite. The two species are found to form a series of stoichiometric complexes of the type AB, A2B, and A 3B on the acid rich side of the phase diagram. Interestingly, no similar series of complexes is evident on the amine rich side. As a result of this complexation, the solid monolayers of the binary mixtures exhibit a very pronounced enhancement in stability relative to the pure adsorbates.
Abril, 2011 | DOI: 10.1021/la1048198
Nanotecnología en Superficies y Plasma
Novel guests for porous columnar thin films: The switchable perchlorinated trityl radical derivatives
Oliveros, M; Gonzalez-Garcia, L; Mugnaini, V; Yubero, F; Roques, N; Veciana, J; Gonzalez-Elipe, AR; Rovira, CLangmuir, 27 (2011) 5098-5106 DOI: 10.1021/la200470f
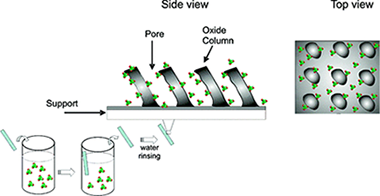
Abstract
TiO2 and SiO2 porous thin films consisting of tilted nanocolumns prepared by glancing angle evaporation (GLAD) have been infiltrated with guest derivatives belonging to the family of perchlorinated trityl radicals, novel guest molecules presenting an open-shell electronic configuration associated with paramagnetism, fluorescence, and electroactivity. The main driving forces for infiltration from aqueous solutions of the carboxylate-substituted radical derivatives are the electrostatic interactions between their negative charge and the net positive charges induced on the film pores. Positive charges on the internal surface of the films were induced by either adjusting the radical solution pH at values lower than the point of zero charge (PZC) of the oxide or passivating the nanocolumns oxide surface with a positively charged aminosilane. The infiltrated composite thin films are robust and easy to handle thanks to the physical protection exerted by the film columns. They also keep the multifunctionality of the used guests, as confirmed by electron paramagnetic resonance (EPR), UV-vis spectroscopy, and fluorescence spectroscopy. To prove the electroactivity of the infiltrated porous films, a porous TiO2 host layer was supported onto conductive indium tin oxide (ITO). By application of an appropriate redox potential, the guest radical molecules have been reversibly switched from their open-shell electronic configuration to their diamagnetic state and hence changed their optical properties. On the basis of these results, it is herein proposed that the appropriate surface functionalization of the pore internal surface of GLAD thin films can be used to prepare novel radical-oxide composite thin films usable for the development of robust switchable electrically driven photonic and magnetic devices.
Abril, 2011 | DOI: 10.1021/la200470f
Materiales y Procesos Catalíticos de Interés Ambiental y Energético - Fotocatálisis Heterogénea: Aplicaciones
Photodeposition of gold on titanium dioxide for photocatalytic phenol oxidation
Hidalgo, MC; Murcia, JJ; Navio, JA; Colon, GApplied Catalysis A-General, 397 (2011) 112-120 DOI: 10.1016/j.apcata.2011.02.030
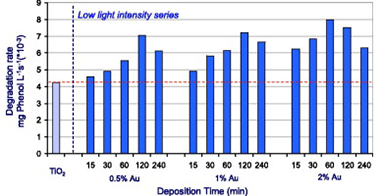
Abstract
The influence of experimental conditions during the photodeposition in the preparation of supported Au on TiO2 has been studied. Besides preparation pH, light intensity and deposition time showed to have a high influence on the final properties of gold deposits. Photodeposition using illumination with a high light intensity UV-vis lamp (140 W/m2 UVA range) resulted to be an ineffective method for obtaining nanoparticles of gold on the titania, producing very large and poorly distributed gold deposits. Thus obtained materials did not show any important improvement of their photocatalytic activity tested for phenol oxidation. By contrast, photodeposition using a low light intensity of illumination (0.15 W/m 2 UVA range), produced materials with notably improved photocatalytic activity. The illumination with such a low light intensity allowed the control of the amount, aggregation and oxidation state of gold by changing deposition time, enabling a feasible method of tailoring Au-TiO2 with the appropriate properties for a high photocatalytic activity. Best photocatalytic behaviour for phenol photodegradation was obtained for Au-TiO2 samples prepared by photodeposition at low light intensity with 120 min photodeposition time for catalysts with a 0.5% and 1% nominal content of gold and with 60 min photodeposition time for catalyst with a 2% nominal content of gold.
Abril, 2011 | DOI: 10.1016/j.apcata.2011.02.030
Nanotecnología en Superficies y Plasma
Rhodamine 6G and 800 J-heteroaggregates with enhanced acceptor luminescence (HEAL) adsorbed in transparent SiO2 GLAD thin films
Sanchez-Valencia, JR; Aparicio, FJ; Espinos, JP; Gonzalez-Elipe, AR; Barranco, APhysical Chemistry Chemical Physics, 13 (2011) 7071-7082 DOI: 10.1039/c0cp02421j
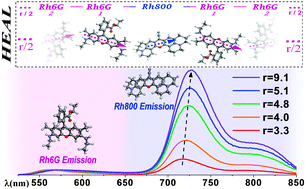
Abstract
An enhanced fluorescent emission in the near infrared is observed when the Rhodamine 800 (Rh800) and 6G (Rh6G) dyes are coadsorbed in porous SiO 2 optical thin films prepared by glancing angle deposition (GLAD). This unusual behavior is not observed in solution and it has been ascribed to the formation of a new type of J-heteroaggregates with enhanced acceptor luminescence (HEAL). This article describes in detail and explains the main features of this new phenomenology previously referred in a short communication [J. R. Sánchez-Valencia, J. Toudert, L. González-García, A. R. González-Elipe and A. Barranco, Chem. Commun., 2010, 46, 4372-4374]. It is found that the efficiency and characteristics of the energy transfer process are dependent on the Rh6G/Rh800 concentration ratio which can be easily controlled by varying the pH of the solutions used for the infiltration of the molecules or by thermal treatments. A simple model has been proposed to account for the observed enhanced acceptor luminescence in which the heteroaggregates order themselves according to a "head to tail" configuration due to the geometrical constrains imposed by the SiO2 porous matrix thin film. The thermal stability of the dye molecules within the films and basic optical (absorption and fluorescence) principles of the HEAL process are also described.
Abril, 2011 | DOI: 10.1039/c0cp02421j
Materiales Coloidales
Tuning from blue to magenta the up-converted emissions of YF3:Tm3+/Yb3+ nanocrystals
Quintanilla, M; Nunez, NO; Cantelar, E; Ocana, M; Cusso, FNanoscale, 3 (2011) 1046-1052 DOI: 10.1039/c0nr00676a
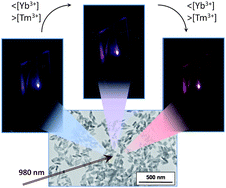
Abstract
Monodisperse YF3:Tm3+/Yb3+ nanocrystals have been synthesized to explore the visible up-converting properties under near infrared (975 nm) excitation. It has been found that the nanoparticles exhibit intense red up-converted emissions, in addition to the characteristic UV and blue Tm3+-bands. It is demonstrated that, by carefully selecting Tm3+ and Yb3+ contents, the relative intensity of the different emissions can be changed producing an overall emission colour that can be tuned from blue to magenta.
Marzo, 2011 | DOI: 10.1039/c0nr00676a
Reactividad de Sólidos
Mechanochemical synthesis of visible light sensitive titanium dioxide photocatalyst
Šubrt, J., Criado, J.M., Szatmáry, L., Diánez-Millán, M.J., Murafa, N., Pérez-Maqueda, L.A., Brezová, V.International Journal of Photoenergy, 2011 (2011) Article number 156941 DOI: 10.1155/2011/156941

Abstract
Phase transition of anatase nanoparticles into the phases TiO2-II and rutile under grinding was studied. The addition of ammonium carbamate to the reaction mixture inhibits the phase conversion and the cold welding of particles. The UV-visible absorption spectrum showed narrowing the band gap width after grinding with an ammonium carbamate additive resulting in shift of the light absorption of the ground sample towards the visible region. By EPR, intensive formation of OH• radical at irradiation of the sample with both UV (λ > 300 nm) and visible (λ > 435 nm) light was observed. High photocatalytic activity of the ground sample in visible light region was demonstrated also by measurement of kinetics of the photocatalytic decomposition of 4-chlorophenol.
Marzo, 2011 | DOI: 10.1155/2011/156941
Materiales Nanoestructurados y Microestructura
A comparative study of the role of additive in the MgH2 vs. the LiBH4–MgH2 hydrogen storage system
A. Fernández, E. Deprez, O. FriedrichsInternational Journal of Hydrogen Energy, 36 (2011) 3932-3940 DOI: 10.1016/j.ijhydene.2010.12.112
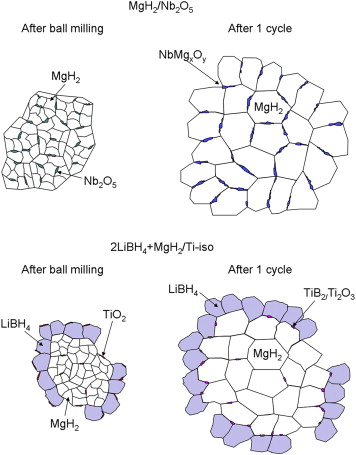
Abstract
The objective of the present work is the comparative study of the behaviour of the Nb- and Ti-based additives in the MgH2 single hydride and the MgH2 + 2LiBH4 reactive hydride composite. The selected additives have been previously demonstrated to significantly improve the sorption reaction kinetics in the corresponding materials. X-Ray Diffraction (XRD), X-Ray Absorption Spectroscopy (XAS), X-Ray Photoelectron Spectroscopy (XPS) and Electron Microscopy (TEM) analysis were carried out for the milled and cycled samples in absence or presence of the additives. It has been shown that although the evolution of the oxidation state for both Nb- and Ti-species are similar in both systems, the Nb additive is performing its activity at the surface while the Ti active species migrate to the bulk. The Nb-based additive is forming pathways that facilitate the diffusion of hydrogen through the diffusion barriers both in desorption and absorption. For the Ti-based additive in the reactive hydride composite, the active species are working in the bulk, enhancing the heterogeneous nucleation of MgB2 phases during desorption and producing a distinct grain refinement that favours both sorption kinetics. The results are discussed in regards to possible kinetic models for both systems.
Marzo, 2011 | DOI: 10.1016/j.ijhydene.2010.12.112
Química de Superficies y Catálisis
Selective CO removal over Au/CeFe and CeCu catalysts in microreactors studied through kinetic analysis and CFD simulations
Arzamendi, G; Uriz, I; Dieguez, PM; Laguna, OH; Hernandez, WV; Alvarez, A; Centeno, MA; Odriozola, JA; Montes, M; Gandia, LMChemical Engineering Journal, 167 (2011) 588-596 DOI: 10.1016/j.cej.2010.08.083
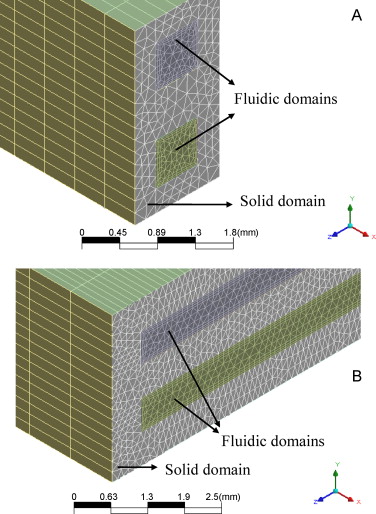
Abstract
A kinetic study of the preferential oxidation of CO in H2 rich streams (CO-PrOx) over a cerium-copper oxide (CeCu) and a gold catalyst supported on cerium-iron oxide (Au/CeFe) is presented. The gold catalyst is very active but the CeCu oxide is more selective. A kinetic model describing the CO-PrOx system with CO2 and H2O in the feed has been formulated considering the oxidation of CO and H2 and the reverse water-gas shift reaction. The rate equations have been implemented in computational fluid dynamics codes to study the influence of the operating variables on the CO-PrOx in microchannels and microslits. The CeCu catalyst is the only one capable of achieving final CO contents below 10-100ppmv. Due to the opposite effect of temperature on activity and selectivity there is an optimal temperature at which the CO content is minimal over CeCu. This temperature varies between 170 and 200°C as the GHSV increases from 10,000 to 50,000h-1. Simulations have evidenced the very good heat transfer performance of the microdevices showing that the CO-PrOx temperature can be controlled using air as cooling fluid although the inlet temperature and flow rate should be carefully controlled to avoid reaction extinction. Both microchannels and microslits behaved similarly. The fact that the microslits are much easier to fabricate may be an interesting advantage in favour of that geometry in this case. © 2010 Elsevier B.V.
Marzo, 2011 | DOI: 10.1016/j.cej.2010.08.083
Materiales Nanoestructurados y Microestructura
Endurance of TiAlSiN coatings: Effect of Si and bias on wear and adhesion
Philippon, D; Godinho, V; Nagy, PM; Delplancke-Ogletree, MP; Fernandez, AWear, 270 (2011) 541-549 DOI: 10.1016/j.wear.2011.01.009
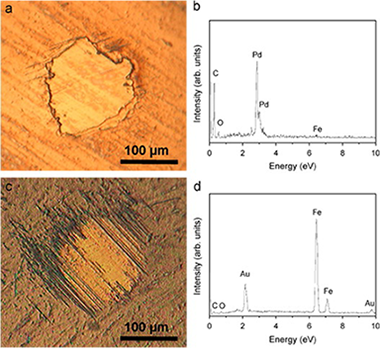
Abstract
In this work, the endurance of TiAlSiN nanocomposite thin films subjected to tribological solicitation is studied. These coating were deposited on M2 steel substrate by magnetron sputtering. Dry sliding experiments were conducted at ambient temperature against WC-Co ball. Coefficients of friction, wear rates and endurances were correlated with the composition, microstructure, mechanical properties, residual stress and adhesion of the coatings. The hardness and elastic modulus were found dependent not only on the composition but also on the residual stress induced by the deposition process. Friction coefficient was found to be independent on Si content while the wear rate is strongly reduced for higher Si contents. The formation of a nanocomposite microstructure, the amount of amorphous Si-based phase and both, wear resistance and adhesion are shown as the critical factors to determine the endurance of the coating.
Marzo, 2011 | DOI: 10.1016/j.wear.2011.01.009
Química de Superficies y Catálisis
Fischer-Tropsch synthesis in microchannels
Almeida, LC; Echave, FJ; Sanz, O; Centeno, MA; Arzamendi, G; Gandia, LM; Sousa-Aguiar, EF; Odriozola, JA; Montes, MChemical Engineering Journal, 167 (2011) 536-544 DOI: 10.1016/j.cej.2010.09.091
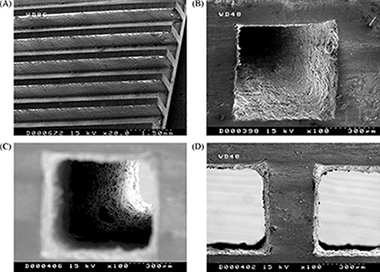
Abstract
Different metallic supports (aluminum foams of 40ppi, honeycomb monolith and micromonolith of 350 and 1180cpsi, respectively) have been loaded with a 20%Co-0.5%Re/γ-Al2O3 catalyst by the washcoating method. Layers of different thicknesses have been deposited onto the metallic supports. The catalytic coatings were characterized measuring their textural properties, adhesion and morphology. These structured catalysts have been tested in the Fischer-Tropsch synthesis (FTS) and compared with a microchannel block presenting perpendicular channels for reaction and cooling. The selectivity depends on the type of support used and mainly on the thickness of the layer deposited. In general, the C5+ selectivity decreased at increasing CO conversion for all of the systems (powder, monoliths, foams and microchannels block). On the other hand, the selectivity to methane increased with the thickness of the catalytic layer due to the higher effective H2/CO ratio over the active sites resulting from the higher diffusivity of H2 compared with CO in the liquid products filling the pores. The C5+ selectivity of the microchannels reactor is higher than that of the structured supports and the powder catalyst. © 2010 Elsevier B.V.
Marzo, 2011 | DOI: 10.1016/j.cej.2010.09.091
Materiales de Diseño para la Energía y Medioambiente
Monolayer structures of alkyl aldehydes: Odd-membered homologues
Phillips, TK; Clarke, SM; Bhinde, T; Castro, MA; Millan, C; Medina, SThin Solid Films, 519 (2011) 3123-3127 DOI: 10.1016/j.tsf.2010.12.084
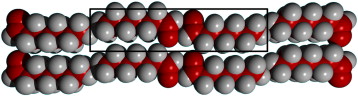
Abstract
Crystalline monolayers of three aldehydes with an odd number of carbon atoms in the alkyl chain (C7, C9 and C11) at low coverages are observed by a combination of X-ray and neutron diffraction. Analysis of the diffraction data is discussed and possible monolayer crystal structures are proposed; although unique structures could not be ascertained for all molecules. We conclude that the structures are flat on the surface, with the molecules lying in the plane of the layer. The C11 homologue is determined to have a plane group of either p2, pgb or pgg, and for the C7 homologue the p2 plane group is preferred.
Marzo, 2011 | DOI: 10.1016/j.tsf.2010.12.084
Materiales de Diseño para la Energía y Medioambiente
The biophysical design of plant cuticles: an overview
Dominguez, E; Heredia-Guerrero, JA; Heredia, ANew Phytologist, 189 (2011) 938-949 DOI: 10.1111/j.1469-8137.2010.03553.x
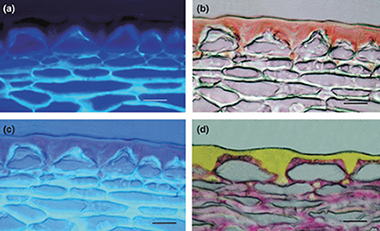
Abstract
The outer surfaces of epidermal cell walls are impregnated with an extracellular matrix called the cuticle. This composite matrix provides several functions at the interface level that enable plants to thrive in different habitats and withstand adverse environmental conditions. The lipid polymer cutin, which is the main constituent of the plant cuticle, has some unique biophysical properties resulting from its composition and structure. This review summarizes the progress made towards understanding the biophysical significance of this biopolymer with special focus on its structural, thermal, biomechanical, and hydric properties and relationships. The physiological relevance of such biophysical properties is discussed in light of existing knowledge on the plant cuticle.
Marzo, 2011 | DOI: 10.1111/j.1469-8137.2010.03553.x
Nanotecnología en Superficies y Plasma
Nitridation of nanocrystalline TiO2 thin films by treatment with ammonia
Romero-Gomez, P; Rico, V; Espinos, JP; Gonzalez-Elipe, AR; Palgrave, RG; Egdell, RGThin Solid Films, 519 (2011) 3587-3595 DOI: 10.1016/j.tsf.2011.01.267
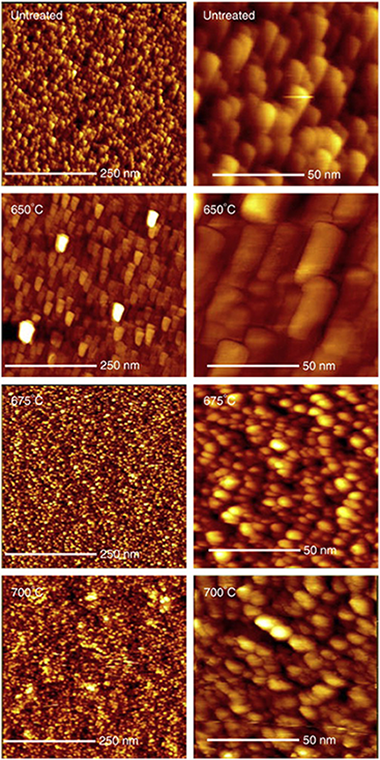
Abstract
Nanocrystalline anatase (TiO2) thin films prepared by a physical vapour deposition method were nitrided by annealing in flowing NH3 at temperatures ranging between 650 °C and 700 °C. It was established that there was a narrow window of temperatures which allowed both incorporation of interstitial nitrogen into the films with retention of the anatase phase without chemical reduction and preservation of the characteristic nanocrystalline morphology. These optimally modified films responded to visible light in photowetting tests and showed the ability to degrade an organic dye under visible light irradiation.
Marzo, 2011 | DOI: 10.1016/j.tsf.2011.01.267
Química de Superficies y Catálisis
Gold nanoparticles on yttrium modified titania: Support properties and catalytic activity
Plata, JJ; Marquez, AM; Sanz, JF; Avellaneda, RS; Romero-Sarria, F; Dominguez, MI; Centeno, MA; Odriozola, JATopics in Catalysis, 54 (2011) 219-228 DOI: 10.1007/s11244-011-9639-4
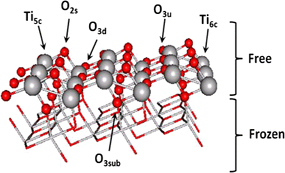
Abstract
A series of titanium oxide catalysts modified with yttrium has been prepared by sol-gel method and their structural properties have been studied. The incorporation of yttrium in the titania lattice favors the formation of oxygen vacancies while at low Y loadings the anatase structure is preserved. The catalytic activity of these solids for CO oxidation is found to be significantly dependent on their physical properties. In particular the amount of dopant controls the number of surface oxygen vacancies created as well as the gold particle size, which directly affects the catalytic activity. Also, a linear relationship between the catalytic activity and the band gap values, which depend on the Y loading, is observed. Density functional theory based calculations show that Y atoms are incorporated at the TiO2 surface at substitutional positions only, while the preferred oxygen vacancies arise by removing the bridge surface oxygen atoms. These O-vacancies are the preferential adsorption sites for Au atoms and nanoparticles, acting as nucleation centers that favor the dispersion of the catalyst active phase over the support surface. In agreement with experiment, Y doping is found to decrease the band gap of the support due to a destabilization of the valence band of the oxide. © 2011 Springer Science+Business Media, LLC 2011.
Marzo, 2011 | DOI: 10.1007/s11244-011-9639-4
Química de Superficies y Catálisis
Creep behavior of TiCxN1-x-CoTi cermets synthesized by mechanically induced self-sustaining reaction
Morales-Rodriguez, A; Gallardo-Lopez, A; Dominguez-Rodriguez, A; Cordoba, JM; Aviles, MA; Gotor, FJJournal of the European Ceramic Society, 31 (2011) 299-302 DOI: 10.1016/j.jeurceramsoc.2010.10.007
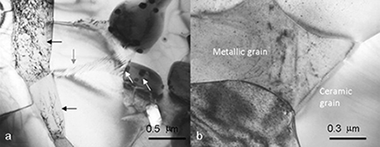
Abstract
The plastic flow of TiCxN1-x-CoTi cermets has been investigated by uniaxial compression tests carried out in argon atmosphere at temperatures between 1100 and 1200°C. Two different cermets, with 5wt.% W or WC content as sintering additives, have been explored to assess the influence of the sintering additives on creep. The microstructural observations of deformed samples and the mechanical results indicate that the hard phase (ceramic grains) controls the plastic deformation. The stress exponent changes from 1 to 2 with increasing strain rate, suggesting a transition in the deformation mechanism from diffusional creep to grain boundary sliding; both with similar activation energy values of about 400kJ/mol. This value of activation energy agrees with C diffusion in the carbonitride grains as the strain rate controlling mechanism.
Marzo, 2011 | DOI: 10.1016/j.jeurceramsoc.2010.10.007
Reactividad de Sólidos - Química de Superficies y Catálisis
Design and testing of a microchannel reactor for the PROX reaction
Cruz, S; Sanz, O; Poyato, R; Laguna, OH; Echave, FJ; Almeida, LC; Centeno, MA; Arzamendi, G; Gandia, LM; Souza-Aguiar, EF; Montes, M; Odriozola, JAChemical Engineering Journal, 167 (2011) 634-642 DOI: 10.1016/j.cej.2010.08.088
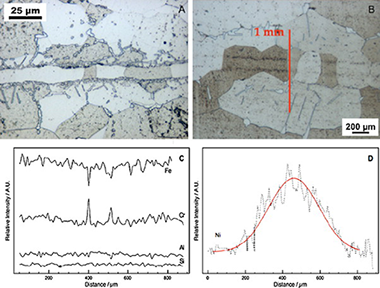
Abstract
The different steps for manufacturing a microchannel reactor for the PROX reaction are discussed. Transient Liquid Phase bonding (TLP) using a Ni-B-Si amorphous melt spun is used for joining micromilled Al-alloyed ferritic stainless steel plates followed by recrystallization at 1200°C for 5h. A CuOx-CeO2 catalyst synthesized by the coprecipitation method was washcoated on the microchannel block resulting in a homogenous 20-30μm thick layer. The catalytic activity for CO-PROX reaction is similar in both the powder catalyst and the microchannel coated reactor but the selectivity is higher in the microchannel reactor. © 2010 Elsevier B.V.
Marzo, 2011 | DOI: 10.1016/j.cej.2010.08.088
Reactividad de Sólidos
Kinetic Analysis of Complex Solid-State Reactions. A New Deconvolution Procedure
Antonio Perejón, Pedro E. Sánchez-Jiménez, José M. Criado, and Luis A. Pérez-MaquedaJournal of Physical Chemistry B, 2011, 115 (8), pp 1780–1791 DOI: 10.1021/jp110895z
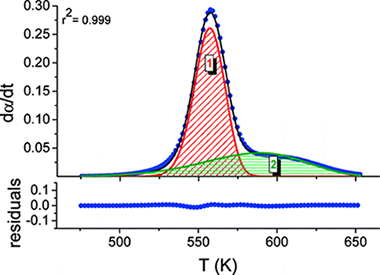
Abstract
The kinetic analysis of complex solid-state reactions that involve simultaneous overlapping processes is challenging. A method that involves the deconvolution of the individual processes from the overall differential kinetic curves obtained under linear heating rate conditions, followed by the kinetic analysis of the discrete processes using combined kinetic analysis, is proposed. Different conventional mathematical fitting functions have been tested for deconvolution, paying special attention to the shape analysis of the kinetic curves. It has been shown that many conventional mathematical curves such as the Gaussian and Lorentzian ones fit kinetic curves inaccurately and the subsequent kinetic analysis yields incorrect kinetic parameters. Alternatively, other fitting functions such as the Fraser-Suzuki one properly fit the kinetic curves independently of the kinetic model followed by the reaction and their kinetic parameters, and moreover, the subsequent kinetic analysis yields the correct kinetic parameters. The method has been tested with the kinetic analysis of complex processes, both simulated and experimental.
Marzo, 2011 | DOI: 10.1021/jp110895z
Materiales de Diseño para la Energía y Medioambiente
Blocking of grain reorientation in self-doped alumina materials
Suarez, M; Fernandez, A; Menendez, JL; Ramirez-Rico, J; Torrecillas, RScripta Materialia, 64 (2011) 517-520 DOI: 10.1016/j.scriptamat.2010.11.031
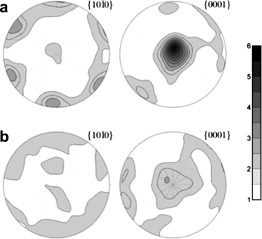
Abstract
Alumina nanoparticles 10-20 nm in diameter were nucleated on alumina particles, 150 nm average diameter, by a colloidal route followed by calcination. It is shown that after sintering, the final grain size is up to 20% smaller due to the addition of the alumina nanoparticles. Electron backscattered diffraction analysis shows that whereas a correlation in the relative crystalline orientations between neighbouring grains exists in the pure materials, the addition of alumina nanoparticles results in a random crystalline orientation.
Marzo, 2011 | DOI: 10.1016/j.scriptamat.2010.11.031
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
Effect of thermal treatments on the catalytic behaviour in the CO preferential oxidation of a CuO-CeO2-ZrO2 catalyst with a flower-like morphology
Moretti, E; Storaro, L; Talon, A; Lenarda, M; Riello, P; Frattini, R; de Yuso, MDM; Jimenez-Lopez, A; Rodriguez-Castellon, E; Ternero, F; Caballero, A; Holgado, JPApplied Catalysis B-Environmental, 102 (2011) 627-637 DOI: 10.1016/j.apcatb.2011.01.004

Abstract
A Ce–Zr–Cu oxide system with a flower-like morphology was prepared by a slow co-precipitation method in the absence of any structure directing agent. Four portions of the oxide were thermally treated at four different temperatures (350 °C, 450 °C, 550 °C, 650 °C). The resulting materials samples were characterized by quantitative XRD, adsorption–desorption of N2 at-196 °C, SEM and TEM microscopy, –H2-TPR, XPS and Operando-XANES. All samples were tested in the preferential CO oxidation (CO-PROX) in the 40–190 °C temperature range. Thermal treatments were found to induce slight structural changes without altering the starting morphology of the samples. The samples treated at higher temperature 550–650 °C showed a quite interesting CO-PROX activity and selectivity in a temperature range suitable for a practical use within the FEMFC technology.
Febrero, 2011 | DOI: 10.1016/j.apcatb.2011.01.004
Química de Superficies y Catálisis
Aluminum anodization in oxalic acid: Controlling the Texture of Al 2O3/Al monoliths for catalytic aplications
Sanz, O; Echave, FJ; Odriozola, JA; Montes, MIndustrial and Engineering Chemistry Research, 50 (2011) 2117-2125 DOI: 10.1021/ie102122x
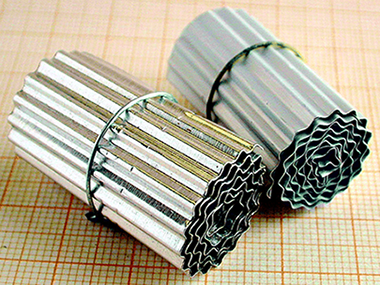
Abstract
The anodization and postanodization processes of aluminum in order to prepare monoliths for catalytic applications have been studied in this work using oxalic acid as electrolyte. The effect of anodization variables (anodization time, current density, temperature, and electrolyte concentration) and postanodization processes on the surface morphology and textural properties of AAO (anodic aluminum oxide) films is analyzed. The anodization variables affect the two main processes taking part in the Al2O3 layer formation: alumina generation and its dissolution that are controlled by temperature, electrolyte concentration and time. The proper combination of both processes, as a result of the anodization variables choice, produces adherent alumina layers with tailored porosity and surface morphology that show excellent properties to be used as catalyst structured support. Larger pore sizes and the complete absence of sulfur that may poison reduced metal-supported active phases are main differences with the classical, most often used, sulfuric acid anodization process. © 2011 American Chemical Society.
Febrero, 2011 | DOI: 10.1021/ie102122x
Materiales de Diseño para la Energía y Medioambiente
Examination of competitive lanthanide sorption onto smectites and its significance in the management of radioactive waste
Galunin, E; Alba, MD; Santos, MJ; Abrao, T; Vidal, MJournal of Hazardous Materials, 186 (2011) 1930-1941 DOI: 10.1016/j.jhazmat.2010.12.098
Abstract
The competitive effect of La and Lu (analogues of radionuclides appearing in radioactive waste) in the sorption in four smectites was examined. Sorption and desorption distribution coefficients (Kd; Kd,des), and desorption rates (Rdes) were determined from batch tests in two media: deionized water and, to consider the influence of cement leachates, 0.02molL-1 Ca. The competitive effect was lower when high-affinity sites were available, as in the water medium at the lowest range of initial lanthanide concentration, with high Kd for La and for Lu (5-63×104Lkg-1). Lower Kd was measured at higher initial concentrations and in the Ca medium, where Lu showed a stronger competitive effect. This was confirmed by fitting the sorption data to a two-solute Langmuir isotherm. The desorption data indicated that sorption was virtually irreversible for the scenarios with high sorption, with an excellent correlation between Kd and Kd,des (R2 around 0.9 for the two lanthanides). Assuming that radioactive waste is a mixture of radionuclides, and that Ca ions will be provided by the cement leachates, this would reduce the retention capacity of clay engineered barriers.
Febrero, 2011 | DOI: 10.1016/j.jhazmat.2010.12.098
Intercalation and dynamics of hydrated Fe2+ in the vermiculites from Santa Olalla and Ojén
Anton Lerf, Friedrich E. Wagner, Juan Poyato and José Luis Pérez-RodríguezJournal of Solid State Electrochemistry, 15 (2011) 223-229 DOI: 10.1007/s10008-010-1171-0
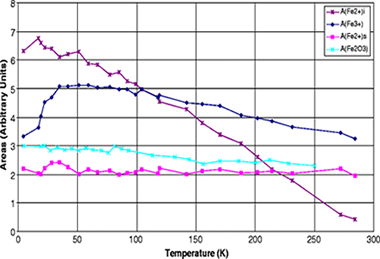
Abstract
Although the intercalation of Fe3+ into layered phyllosicilicates-especially into smectites-attracted much attention in the past two decades, the information about Fe2+ loaded phyllosilicates is sparse. Here we present an investigation of the Fe2+ exchanged vermiculites from Santa Olalla and Ojén (Andalusia, Spain) by means of Mössbauer spectroscopy. The room temperature Mössbauer spectra are very similar to those of the starting compounds (Na forms) except for a decrease of the contribution of structural Fe3+ and a concomitant increase of the contribution of Fe2+ sites, indicating an internal redox process. The extent of this redox reaction is different for the two vermiculites. Thus, the intercalated Fe2+ acts as an electron mediator from the external medium to the structural Fe3+ ions. A new component attributable to intercalated Fe2+ is practically invisible in the room temperature Mössbauer spectra, but increases strongly and continuously during cooling to 4.2 K, where it is the dominant feature of the Mössbauer patterns. At 4.2 K, its quadruple splitting amounts to 3.31 mm/s, which is in excellent agreement with the quadrupole slitting of Fe2+ coordinated to six water molecules in a highly symmetric octahedral arrangement. The strong decrease of the Mössbauer-Lamb factor of this component with increasing temperature indicates a weak bonding of the Fe 2+ in the interlayer space.
Febrero, 2011 | DOI: 10.1007/s10008-010-1171-0
Nanotecnología en Superficies y Plasma
Nitrogen plasma functionalization of low density polyethylene
Lopez-Santos, C; Yubero, F; Cotrino, J; Gonzalez-Elipe, ARSurface and Coatings Technology, 205 (2011) 3356-3365 DOI: 10.1016/j.surfcoat.2010.11.038
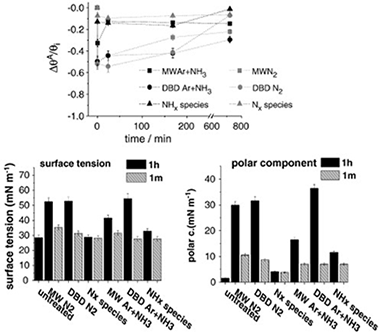
Abstract
Low density polyethylene (LDPE) films have been treated with different nitrogen containing plasmas with the purpose of incorporating nitrogen functional groups on its surface and analyzing the changes experienced in their surface tension. Effects of a dielectric barrier discharge (DBD) at atmospheric pressure and a microwave discharge (MW) at reduced pressure are compared with those obtained by using an atom source supplied with N2 and mixtures Ar+NH3 as plasma gas. X-ray photoelectron spectroscopy (XPS) analysis has provided information about the chemical surface changes whereas the surface topography of the treated samples has been examined by atomic force microscopy (AFM). Non-destructive depth profiles of oxygen and carbon have been obtained for the treated and one month aged samples by means of the non-destructive Tougaard's method of XPS background analysis. Generally, an oxygen enrichment of the deeper region of treated LDPE surfaces has been observed. Chemical derivatization of the treated samples has shown that a DBD plasma with a mixture of Ar+NH3 was the most efficient treatment for nitrogen and amine group functionalization. It is argued that the high concentration of NH* species in this plasma is the most important factor in enhancing the nitrogen functionalization of this polymer. It has been also found that the observed increase in hydrophilicity and surface tension cannot be attributed to the anchored nitrogen functional groups formed on plasma treated LDPE. Differences in the plasma activation behaviour of LDPE and that of other polymers subjected to similar treatments are stressed.
Febrero, 2011 | DOI: 10.1016/j.surfcoat.2010.11.038
Study of ground and unground leached vermiculite II. Thermal behaviour of ground acid-treated vermiculite
Perez-Rodriguez, JL; Maqueda, C; Murafa, N; Subrt, J; Balek, V; Pulisova, P; Lancok, AApplied Clay Science, 51 (2011) 274-282 DOI: 10.1016/j.clay.2010.11.031
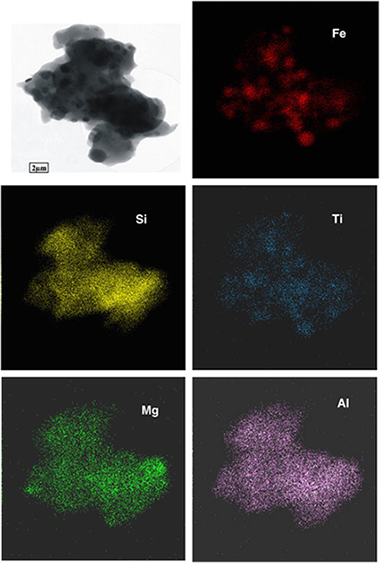
Abstract
In this study, we examined the annealing effect on the material obtained after acid treatment of ground vermiculite which constituted amorphous silica and β-FeOOH. The XRD patterns of the starting sample measured at temperatures from 30 to 1200°C showed that the crystalline phase was present until ~300°C; whereas the sample heated between 300 and 800°C was practically amorphous. This is in agreement with previous observations that β-FeOOH decomposes to amorphous or poorly crystalline phase, β-Fe2O3, and transforms only slowly to crystalline α-Fe2O3. At 850°C the sample showed the first signs of a crystalline phase which was fully developed at 1050°C. The XRD, HRTEM and Mössbauer spectroscopy showed, after heating at 1050°C, the presence of crystalline phase, consisting of quartz, cristobalite, α-Fe2O3 and ε-Fe2O3. This effect showed in fact that well crystallized iron oxide nanoparticles embedded into the silica matrix are usually formed at relative high temperatures (~1000°C), which is in contrast to silica-free material. Element mapping of one particle of the composite obtained by annealing the sample at the highest temperature showed well-separated Fe2O3 and SiO2 particles in a composite material. Impurities of Al and Mg (from the original vermiculite) accompanied the silica components and TiO2 associated with Fe2O3 grains was also detected.
Febrero, 2011 | DOI: 10.1016/j.clay.2010.11.031
Nanotecnología en Superficies y Plasma
Lateral and in-depth distribution of functional groups on diamond-like carbon after oxygen plasma treatments
Lopez-Santos, C; Yubero, F; Cotrino, J; Gonzalez-Elipe, ARDiamond and Related Materials, 20 (2011) 49-56 DOI: 10.1016/j.diamond.2010.11.024
Abstract
A diamond like carbon material has been exposed to a low pressure microwave and atmospheric pressure plasma of oxygen to enhance its hydrophilicity and surface energy. For comparison, data are also reported after activation with a beam of neutral atoms of oxygen. The surface incorporation of oxygenated functional groups and the determination of the in-depth distribution of this element have been analysed by means of the X ray photoemission spectroscopy (XPS). Atomic force microscopy (AFM) has been used to get information of the surface topography and, by recording friction maps of the surface, the lateral distribution of oxygenated functional groups formed after the different activation treatments. Differences in surface composition, topography and in-depth and lateral distribution of oxygen have been correlated with the intrinsic characteristics of the activation plasma processes.
Febrero, 2011 | DOI: 10.1016/j.diamond.2010.11.024
Materiales de Diseño para la Energía y Medioambiente
Influence of OH− concentration on the illitization of kaolinite at high pressure
M. Mantovani, A. Escudero, A.I. Becerro,Applied Clay Science, 51 (2011) 220-225 DOI: 10.1016/j.clay.2010.11.021
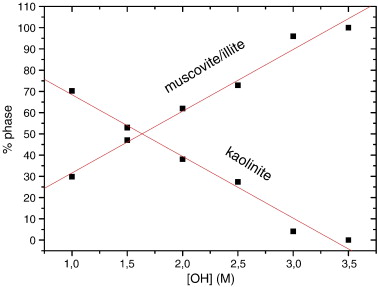
Abstract
The products of hydrothermal reactions of kaolinite at 300 °C and 1000 bars were studied in KOH solutions covering an OH− concentration, [OH−], of 1 M to 3.5 M. XRD patterns indicated a notable influence of the [OH−] on the reaction. At [OH] ≥ 3 M, the only stable phase was muscovite/illite. The content of muscovite/illite was calculated from the analysis of the diagnostic 060 reflections of kaolinite and muscovite/illite. The results showed a linear dependence of kaolinite and muscovite/illite contents with [OH−]. 27Al MAS NMR spectroscopy revealed the formation of small nuclei of K-F zeolite at high [OH−]. Finally, modelling of the 29Si MAS NMR spectra indicated that the Si/Al ratio of the muscovite/illite formed was very close to that of muscovite, at least in the mineral formed at low [OH−]. In good agreement with the XRD data, the quantification of the reaction products by 29Si MAS NMR indicated a linear decrease of the kaolinite content with increasing OH− concentration.
Febrero, 2011 | DOI: 10.1016/j.clay.2010.11.021
Nanotecnología en Superficies y Plasma
Transparent Nanometric Organic Luminescent Films as UV-Active Components in Photonic Structures
Aparicio, FJ; Holgado, M; Borras, A; Blaszczyk-Lezak, I; Griol, A; Barrios, CA; Casquel, R; Sanza, FJ; Sohlstrom, H; Antelius, M; Gonzalez-Elipe, AR; Barranco, AAdvanced Materials, 23 (2011) 761-765 DOI: 10.1002/adma.201003088
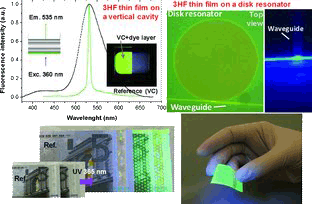
Abstract
A new kind of visible-blind organic thin-film material, consisting of a polymeric matrix with a high concentration of embedded 3-hydroxyflavone (3HF) dye molecules, that absorbs UV light and emits green light is presented. The thin films can be grown on sensitive substrates, including flexible polymers and paper. Their suitability as photonic active components photonic devices is demonstrated.
Febrero, 2011 | DOI: 10.1002/adma.201003088
- ‹ anterior
- 46 of 54
- siguiente ›














