Artículos SCI
2022
2022
Reduction of N2O with hydrosilanes catalysed by RuSNS nanoparticles
Molinillo, P; Lacroix, B; Vattier, F; Rendon, N; Suarez, A; Lara, PChemical Communications
Show abstract ▽
A series of RuSNS nanoparticles, prepared by decomposition of Ru(COD)(COT) with H-2 in the presence of an SNS ligand, have been found to catalyse the reduction of the greenhouse gas N2O to N-2 employing different hydrosilanes.
Junio, 2022 | DOI: 10.1039/d2cc01470j
Materiales Ópticos Multifuncionales
Effect of Spatial Inhomogeneity on Quantum Trapping
Esteso, V; Carretero-Palacios, S; Miguez, HJournal of Physical Chemistry Letters, 13 (2022) 4513-4519
Show abstract ▽
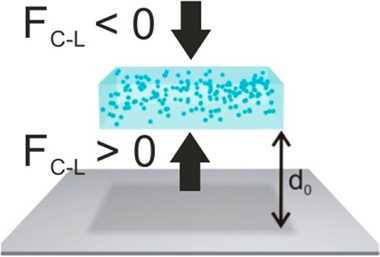
An object that is immersed in afluid and approaching a substrate mayfind apotential energy minimum at a certain distance due to the balance between attractive and repulsiveCasimir-Lifshitz forces, a phenomenon referred to as quantum trapping. This equilibriumdepends on the relative values of the dielectric functions of the materials involved. Herein, westudy quantum trapping effects in planar nanocomposite materials and demonstrate that they arestrongly dependent on the characteristics of the spatial inhomogeneity. As a model case, weconsider spherical particles embedded in an otherwise homogeneous material. We propose aneffective medium approximation that accounts for the effect of inclusions andfind that anunprecedented and counterintuitive intense repulsive Casimir-Lifshitz force arises as a result ofthe strong optical scattering and absorption size-dependent resonances caused by their presence. Our results imply that the properanalysis of quantum trapping effects requires comprehensive knowledge and a detailed description of the potential inhomogeneity(caused by imperfections, pores, inclusions, and density variations) present in the materials involved
Junio, 2022 | DOI: 10.1021/acs.jpclett.2c00807
Nanotecnología en Superficies y Plasma
Titania Enhanced Photocatalysis and Dye Giant Absorption in Nanoporous 1D Bragg Microcavities
Rico, VJ; Turk, H; Yubero, F; González-Elipe, ARACS Applied Nano Materials, 5 (2022) 5487-5497
Show abstract ▽
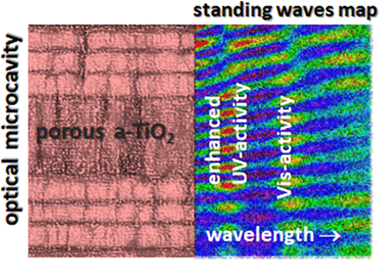
Light trapping effects are known to boost the photocatalytic degradation of organic molecules in 3D photonic structures of anatase titania (a-TiO2) with an inverse opal configuration. In the present work, we show that photocatalytic activity can also be enhanced in a-TiO2 thin films if they are incorporated within a nanoporous 1D optical resonant microcavity. We have designed and manufactured multilayer systems that, presenting a high open porosity to enable a straightforward diffusion of photodegradable molecules, provide light confinement effects at wavelengths around the absorption edge of photoactive a-TiO2. In brief, we have observed that a nanoporous 1D Bragg microcavity prepared by electron beam evaporation at oblique angles comprising a central defect layer of nanoporous a-TiO2 boosts the photocatalytic degradation of nitrobenzene and methyl orange dye solutions. The multilayer structure of the microcavity was designed to ensure the appearance of optical resonances at the a-TiO2 layer location and wavelengths around the absorption onset of this semiconductor. In this porous 1D Bragg microcavity, the diffusion constraints of molecules through the capping layers covering the a-TiO2 are effectively compensated by an increase in the photocatalytic activity due to the light confinement phenomena. We also report that the absorption coefficient of methyl orange dye solution infiltrated within the pore structure of the microcavity is exalted at the wavelengths of the corresponding optical resonances. This effect gives rise to a small but non-negligible visible light photodegradation of dye molecules. The possibilities of tailoring the design of 1D photonic systems to boost the photocatalytic activity of a-TiO2 are discussed.
Junio, 2022 | DOI: 10.1021/acsanm.2c00477
Reactividad de Sólidos
Thermal behavior of ammonium fluorosilicates complexes: Obtaining and kinetic analysis
Resentera, AC; Perejon, A; Esquivel, MR; Perez-Maqueda, LA; Rodriguez, MHChemical Engineering Research amd Design, 182 (2022) 490-501
Show abstract ▽
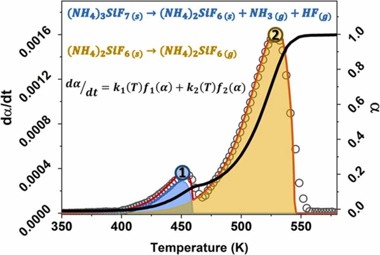
In this work, a mixture of (NH4)3SiF7/(NH4)2SiF6 powders was obtained as a by-product of the Li extraction process from alpha-spodumene aluminosilicate with NH4HF2. The thermal behavior of the powders was analyzed by non-isothermal thermogravimetric experiments. The kinetic parameters that describe the processes involved were obtained using mathematical deconvolution, Friedman's method, combined kinetic analysis, and nonlinear regression optimization. The results show that the process occurs in two partially overlapping steps, the thermal decomposition of (NH4)3SiF7 into (NH4)2SiF6 and the subsequent sublimation of (NH4)2SiF6. The apparent activation energies were 72.6 and 79.8 kJ/ mol for steps 1 and 2, respectively. The apparent pre-exponential factors were 1.19 x 106 and 2.50 x 105 s-1, respectively. The kinetic models indicated that Step 1 follows an A2 model, while Step 2 follows an F0 model. Finally, the resulting kinetic parameters allowed the reconstruction of the original experimental curves and obtaining predictions of curves recorded under other heating programs.
Junio, 2022 | DOI: 10.1016/j.cherd.2022.04.021
Materiales Avanzados
Thermal behaviour of the different parts of almond shells as waste biomass
Garzon, E; Arce, C; Callejon-Ferre, AJ; Perez-Falcon, JM; Sanchez-Soto, PJJournal of Thermal Analysis and Calorimetry, 147 (2022) 5023-5035
Show abstract ▽
The main aim of this study is to investigate the thermal behaviour of the different parts of almond shells produced in an almond industry as a waste biomass. For this purpose, several experiments have been conducted under laboratory conditions. After removing the mature almonds, the waste raw materials subject of this study were treated with distilled water (10 min) and separated in several parts. Taking into account their physical characteristics, they were: (a) complete shells: exocarp, mesocarp and endocarp without grinding (Sample C); (b) ground samples of complete shells, sieved under 0.2 mm (Sample M); (c) hard layers of the endocarp (Sample E); (d) internal layers of the endocarp (Sample I); and (e) mature drupes (Sample P) or skin, being constituted by the flexible part of green colour (fresh form) or yellow (after drying). The thermal behaviour of all these sample materials has been investigated using a laboratory furnace, with determination of ash contents and mass loss by progressive heating (120 min of holding time). Elemental and DTA-TG/DTG analyses of selected sample materials have been carried out. Although a complete study can be very complex, a first approach has been performed in this investigation. Results on thermal decomposition of this biomass waste have been presented to emphasize the main differences between sample materials of almond shells. These results have demonstrated the influence of several parameters, such as the particle size, and previous treatments in the thermal behaviour of the different parts of the almond shells, as showed in this investigation. Structural analysis of almond shells allowed to determine lignin, cellulose and hemicellulose. From the lignin content, it has been predicted the higher heating value (18.24 MJkg(-1)) of this waste as by-product of industrial interest. Other linear correlations to calculate this parameter have been applied with similar results in all these samples.
Junio, 2022 | DOI: 10.1007/s10973-021-10940-x
- ‹ anterior
- 57 of 420
- siguiente ›














