Artículos SCI
2021
2021
Materiales Ópticos Multifuncionales
Self-preserving ice layers on CO2 clathrate particles: Implications for Enceladus, Pluto, and similar ocean worlds
Bostrom, M; Esteso, V; Fiedler, J; Brevik, I; Buhmann, SY; Persson, C; Carretero-Palacios, S; Parsons, DF; Corkey, RWAstronomy & Astrophysics, 650 (2021) A54
Show abstract ▽
Context. Gas hydrates can be stabilised outside their window of thermodynamic stability by the formation of an ice layer - a phenomenon termed self-preservation. This can lead to a positive buoyancy for clathrate particles containing CO2 that would otherwise sink in the oceans of Enceladus, Pluto, and similar oceanic worlds.Aims. Here we investigate the implications of Lifshitz forces and low occupancy surface regions on type I clathrate structures for their self-preservation through ice layer formation, presenting a plausible model based on multi-layer interactions through dispersion forces.Methods. We used optical data and theoretical models for the dielectric response for water, ice, and gas hydrates with a different occupancy. Taking this together with the thermodynamic Lifshitz free energy, we modelled the energy minima essential for the formation of ice layers at the interface between gas hydrate and liquid water.Results. We predict the growth of an ice layer between 0.01 and 0.2 mu m thick on CO, CH4, and CO2 hydrate surfaces, depending on the presence of surface regions depleted in gas molecules. Effective hydrate particle density is estimated, delimiting a range of particle size and compositions that would be buoyant in different oceans. Over geological time, the deposition of floating hydrate particles could result in the accumulation of kilometre-thick gas hydrate layers above liquid water reservoirs and below the water ice crusts of their respective ocean worlds. On Enceladus, the destabilisation of near-surface hydrate deposits could lead to increased gas pressures that both drive plumes and entrain stabilised hydrate particles. Furthermore, on ocean worlds, such as Enceladus and particularly Pluto, the accumulation of thick CO2 or mixed gas hydrate deposits could insulate its ocean against freezing. In preventing freezing of liquid water reservoirs in ocean worlds, the presence of CO2-containing hydrate layers could enhance the habitability of ocean worlds in our Solar System and on the exoplanets and exomoons beyond.
Junio, 2021 | DOI: 10.1051/0004-6361/202040181
Materiales de Diseño para la Energía y Medioambiente
New Trends in Nanoclay-Modified Sensors
Pavon, E; Martin-Rodriguez, R; Perdigon, AC; Alba, MDInorganics, 9 (2021) 43
Show abstract ▽

Nanoclays are widespread materials characterized by a layered structure in the nano-scale range. They have multiple applications in diverse scientific and industrial areas, mainly due to their swelling capacity, cation exchange capacity, and plasticity. Due to the cation exchange capacity, nanoclays can serve as host matrices for the stabilization of several molecules and, thus, they can be used as sensors by incorporating electroactive ions, biomolecules as enzymes, or fluorescence probes. In this review, the most recent applications as bioanalyte sensors are addressed, focusing on two main detection systems: electrochemical and optical methods. Particularly, the application of electrochemical sensors with clay-modified electrodes (CLME) for pesticide detection is described. Moreover, recent advances of both electrochemical and optical sensors based on nanoclays for diverse bioanalytes' detection such as glucose, H2O2, organic acids, proteins, or bacteria are also discussed. As it can be seen from this review, nanoclays can become a key factor in sensors' development, creating an emerging technology for the detection of bioanalytes, with application in both environmental and biomedical fields.
Junio, 2021 | DOI: 10.3390/inorganics9060043
Materiales de Diseño para la Energía y Medioambiente
Structural Evolution in Iron-Catalyzed Graphitization of Hard Carbons
Gomez-Martin, A; Schnepp, Z; Ramirez-Rico, JChemistry of Materials, 33 (2021) 3087-3097
Show abstract ▽
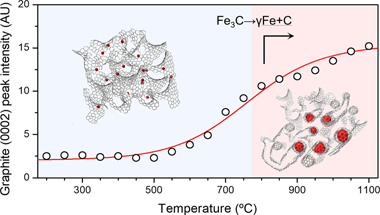
Despite the recent interest in catalytic graphitization to obtain graphite-like materials from hard-carbon sources, many aspects of its mechanism are still poorly unknown. We performed a series of in situ experiments to study phase transformations during graphitization of a hard-carbon precursor using an iron catalyst at temperatures up to 1100 degrees C and ex situ total scattering experiments up to 2000 degrees C to study the structural evolution of the resulting graphitized carbon. Our results show that upon heating and cooling, iron undergoes a series of reductions to form hematite, magnetite, and wustite before forming a carbide that later decomposes into metallic iron and additional graphite and that the graphitization fraction increases with increasing peak temperature. Structural development with temperature results in decreasing sheet curvature and increased stacking, along with a decrease in turbostratic disorder up to 1600 degrees C. Higher graphitization temperatures result in larger graphitic domains without further ordering of the graphene sheets. Our results have implications for the synthesis of novel biomass-derived carbon materials with enhanced crystallinity.
Mayo, 2021 | DOI: 10.1021/acs.chemmater.0c04385
Materiales Avanzados
Synthesis of clay geopolymers using olive pomace fly ash as an alternative activator. Influence of the additional commercial alkaline activator used
Gomez-Casero, MA; Moral-Moral, FJ; Perez-Villarejo, L; Sanchez-Soto, PJ; Eliche-Quesada, DJournal of Materials Research and Technology-JMR&T 12 (2021) 1762-1776
Show abstract ▽
In this research, the use of olive pomace fly ash (OPFA) as an alkaline source for the activation of calcined clays (CC) from Bailen (Jaen, Spain) was studied. The optimal composition was obtained for 70 wt % CC and 30 wt % OPFA. The physical, mechanical and thermal properties of control geopolymers that use water as a liquid medium have been studied and compared with geopolymers that use additional activating solutions as sodium or potassium hydroxide solutions (8 M), or a mixture of alkaline hydroxide and alkaline silicate solution (NaOH-Na2SiO3 or KOH-K2SiO3). The results showed that OPFA can be used as an alkaline activator, showing mechanical properties slightly lower than those obtained when additional alkaline hydroxide activating solutions were used. The best compressive strength was obtained for geopolymers that use alkaline silicates as an activating solution. However, the best thermal insulation properties were obtained for control geopolymers. The microstructural characteristics of the geopolymers were evaluated by means of X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy and Scanning Electron Microscopy (SEM-EDS) that corroborate the formation of geopolymeric gel in all the specimens, being the amount of gel formed greater in samples using commercial potassium activating solutions. These results demonstrate the feasibility of using this type of waste, OPFA, as activating reagents in the manufacture of geopolymers or alkaline activated materials. The manufactured geopolymers can be used as compressed earth blocks for walls and partitions, since the specimens pursue mechanical properties that comply with current regulations, presenting better thermal insulation properties.
Mayo, 2021 | DOI: 10.1016/j.jmrt.2021.03.102
Química de Superficies y Catálisis
Understanding the opportunities of metal-organic frameworks (MOFs) for CO2 capture and gas-phase CO2 conversion processes: a comprehensive overview
Gandara-Loe, J; Pastor-Perez, L; Bobadilla, LF; Odriozola, JA; Reina, TRReaction Chemistry & Engineering, 6 (2021) 787-814
Show abstract ▽

The rapid increase in the concentration of atmospheric carbon dioxide is one of the most pressing problems facing our planet. This challenge has motivated the development of different strategies not only in the reduction of CO2 concentrations via green energy alternatives but also in the capture and conversion of CO2 into value-added products. Metal-organic frameworks (MOFs) are a relatively new class of porous materials with unique structural characteristics such as high surface areas, chemical tunability and stability, and have been extensively studied as promising materials to address this challenge. This comprehensive review identifies the specific structural and chemical properties of MOFs that result in advanced CO2 capture capacities and fairly encouraging catalytic CO2 conversion behaviour. More importantly, we describe an interconnection among the unique properties of MOFs and the engineering aspects of these intriguing materials towards CO2 capture and conversion processes.
Mayo, 2021 | DOI: 10.1039/d1re00034a
- ‹ anterior
- 85 of 420
- siguiente ›














