Artículos SCI
2018
2018
Química de Superficies y Catálisis
Improving the activity of gold nanoparticles for the water-gas shift reaction using TiO2-Y2O3: an example of catalyst design
Plata, JJ; Romero-Sarria, F; Suarez, JA; Marquez, AM; Laguna, OH; Odriozola, JA; Sanz, JFPhysical Chemistry Chemical Physics, 20 (2018) 22076-22083
Show abstract ▽
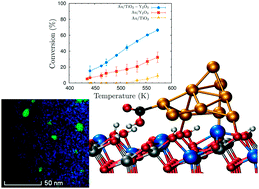
In the last ten years, there has been an acceleration in the pace at which new catalysts for the water-gas shift reaction are designed and synthesized. Pt-based catalysts remain the best solution when only activity is considered. However, cost, operation temperature, and deactivation phenomena are important variables when these catalysts are scaled in industry. Here, a new catalyst, Au/TiO2-Y2O3, is presented as an alternative to the less selective Pt/oxide systems. Experimental and theoretical techniques are combined to design, synthesize, characterize and analyze the performance of this system. The mixed oxide demonstrates a synergistic effect, improving the activity of the catalyst not only at large-to-medium temperatures but also at low temperatures. This effect is related to the homogeneous dispersion of the vacancies that act both as nucleation centers for smaller and more active gold nanoparticles and as dissociation sites for water molecules. The calculated reaction path points to carboxyl formation as the rate-limiting step with an activation energy of 6.9 kcal mol(-1), which is in quantitative agreement with experimental measurements and, to the best of our knowledge, it is the lowest activation energy reported for the water-gas shift reaction. This discovery demonstrates the importance of combining experimental and theoretical techniques to model and understand catalytic processes and opens the door to new improvements to reduce the operating temperature and the deactivation of the catalyst.
Septiembre, 2018 | DOI: 10.1039/c8cp03706j
Química de Superficies y Catálisis - Reactividad de Sólidos
A direct in situ observation of water-enhanced proton conductivity of Eu-doped ZrO2: Effect on WGS reaction
Garcia-Moncada, N; Bobadilla, LF; Poyato, R; Lopez-Cartes, C; Romero-Sarria, F; Centeno, MA; Odriozola, JAApplied Catalysis B-Environmental, 231 (2018) 343-356
Show abstract ▽
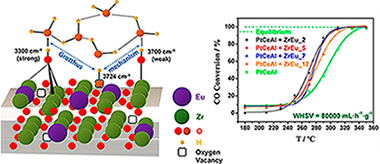
Eu-doped ZrO2 solid solutions have been synthesized in order to prepare proton conductors as water-enhancer additives for the WGS reaction. Elemental characterization has been carried out revealing homogeneous dopant distribution resulting in fluorite-type solid solutions for Eu2O3 contents up to similar to 9 mol.%. Representative samples of the Eu-doped ZrO2 series have been analysed by Impedance Spectroscopy (IS) in inert, oxygen and wet conditions. The solid solution with 5 mol.% of Eu2O3 has presented the highest conductivity values for all tested conditions indicating an optimal amount of dopant. Moreover, the presence of vapour pressure results in an increment of the conductivity at temperatures lower than 300 degrees C, meanwhile at higher temperatures the conductivity is the same than that in inert conditions. To elucidate these results, in situ DRIFTS studies were carried out. These experiments evidenced the existence of water dissociation at oxygen vacancies (band at 3724 cm(-1)) as well as the presence of physisorbed water at temperatures up to similar to 300 degrees C where the band at 5248 cm(-1) characteristic of these species disappeared. These results points to a layer model where the physisorbed water interacts with surface hydroxyls generated by dissociated water that improves the proton conductivity through Grotthuss' mechanism in the RT-300 degrees C temperature range. These samples were successfully tested in WGS reaction as additive to a typical Pt-based catalyst. The presence of the mixed oxide reveals an increase of the catalyst' activity assisted by the proton conductor, since improves the water activation step.
Septiembre, 2018 | DOI: 10.1016/j.apcatb.2018.03.001
Materiales de Diseño para la Energía y Medioambiente
Performance improvement in olive stone's combustion from a previous carbonization transformation
Gomez-Martin, A; Chacartegui, R; Ramirez-Rico, J; Martinez-Fernandez, JFuel, 228 (2018) 254-262
Show abstract ▽
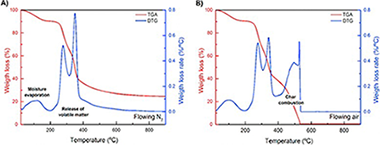
Under the framework of circular economy, agricultural wastes are an interesting carbon-based feedstock for thermal energy and power generation. Their use could extend the availability of biomass-based fuel and, at the same time, would reduce negative environmental effects. However, depending on the residues' characteristics, their direct combustion in boilers presents some challenges which could be overcome with a carbonization pretreatment. In this paper, the main mechanisms of thermochemical transformation of an abundant agricultural waste, olive stone, into biochar products via slow carbonization are analyzed, with emphasis on the effect of peak carbonization temperature. Thermogravimetric and differential scanning calorimetry analysis are used to evaluate the performance of the resulting biochars compared to raw olive stone in combustion processes and to assess the correlation between the peak carbonization temperature and compositional and fuel properties. Results show that with a prior treatment up to an optimum temperature of 800 degrees C the energy density is increased up to three times compared to the raw material. These findings suggest that carbonization of olive stones reduces the barriers to their direct use in current biomass boiler technology.
Septiembre, 2018 | DOI: 10.1016/j.fuel.2018.04.127
Reactividad de Sólidos
Mechanosynthesis of Sr1-xLaxTiO3 anodes for SOFCs: Structure and electrical conductivity
Sayagues, MJ; Gotor, J; Pueyo, M; Poyato, R; Garcia-Garcia, FJJournal of Alloys and Compounds, 763 (2018) 679-686
Show abstract ▽
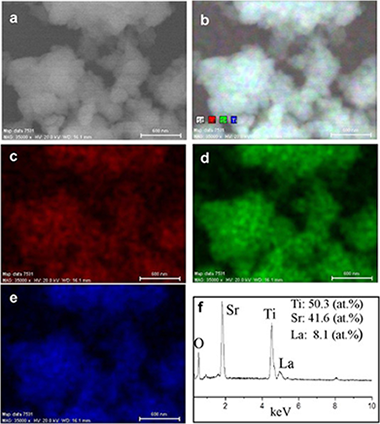
Sr1-xLaxTiO3 (SLT; 0 <= x <= 0.5) powder samples were synthesised at room temperature by a mechanochemical method from SrO, La2O3 and TiO2 mixtures in 90 min. The obtained SLT samples as potential anode materials in solid oxide fuel cells (SOFCs) were investigated. The microstructure, electrical conductivity and chemical compatibility with yttria-stabilised zirconia (YSZ) were studied. The powder samples had a nanometric character after milling. After a subsequently heating at 900 degrees C, the particle size slightly increased, but still remained nanometric. At this high temperature, a good chemical compatibility with YSZ was found. The x = 0.2 sample gave the best electrical conductivity values, i.e. 0.23 W cm(-2). These features make such as-obtained samples good candidates to be used as anodes in SOFCs.
Septiembre, 2018 | DOI: 10.1016/j.jallcom.2018.05.243
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
Solar pilot plant scale hydrogen generation by irradiation of Cu/TiO2 composites in presence of sacrificial electron donors
Maldonado, MI; Lopez-Martin, A; Colon, G; Peral, J; Martinez-Costa, JI; Malato, SApplied Catalysis B-Environmental, 229 (2018) 15-23
Show abstract ▽
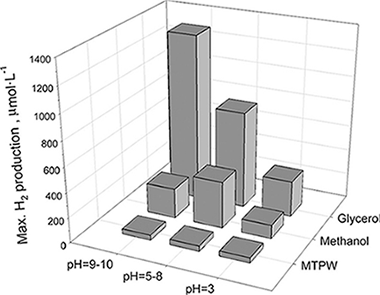
A Cu/TiO2 photocatalyst has been synthesised by reducing a Cu precursor with NaBH4 onto the surface of a sulphate pretreated TiO2 obtained by a sol-gel procedure. The catalyst, that shows a clearly defined anatase phase with high crystallinity and relatively high surface area, and contains Cu2O and CuO deposits on its surface, has been used to produce hydrogen in a solar driven pilot plant scale photocatalytic reactor. Different electron donor aqueous solutions (methanol, glycerol, and a real municipal wastewater treatment plant influent) have been tested showing similar or even higher energy efficiency than those obtained using more expensive noble metal based photocatalytic systems. The glycerol solutions have provided the best reactive environments for hydrogen generation.
Agosto, 2018 | DOI: 10.1016/j.apcatb.2018.02.005
- ‹ anterior
- 158 of 420
- siguiente ›














