Artículos SCI
2015
2015
Química de Superficies y Catálisis
Catalytic screening of Au/CeO2-MOx/Al2O3 catalysts (M = La, Ni, Cu, Fe, Cr, Y) in the CO-PrOx reaction
Reina, TR; Ivanova, S; Centeno, MA; Odriozola, JAInternational Journal of Hydrogen Energy, 40 (2015) 1782-1788
Show abstract ▽
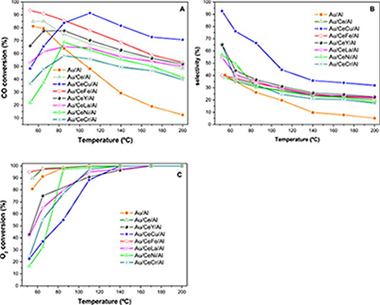
In this work, a series of Au/CeO2-MOx/Al2O3 catalysts has been prepared and evaluated in the PrOx reaction. Within the series of dopants Fe and Cu containing samples enhanced the catalytic performance of the parent Au/CeO2/Al2O3 catalyst being copper the most efficient promoter. For both samples an enhanced oxygen storage capacity (OSC) is registered and accounts for the high CO oxidation activity. More particularly, the Au/CeO2-CuOx/Al2O3 catalyst successfully withstands the inclusion of water in the PrOx stream and presents good results in terms of CO elimination. However to achieve a good selectivity toward, CO2 formation properly adjusting of the reaction parameters, such as oxygen concentration and space velocity is needed. Within the whole screened series the Cu-containing catalyst can be considered as the most interesting alternative for H-2 clean-up applications.
Enero, 2015 | DOI: 10.1016/j.ijhydene.2014.11.141
Reactividad de Sólidos
New Insights on the Kinetic Analysis of Isothermal Data: The Independence of the Activation Energy from the Assumed Kinetic Model
Sanchez-Jimenez, PE; Perejon, A; Perez-Maqueda, LA; Criado, JMEnergy & Fuels, 29 (2015) 392-397
Show abstract ▽
Isothermal experiments are widely employed to study the kinetics of solid-state reactions or processes to extract essential kinetic information needed for modeling the processes at an industrial scale. The kinetic analysis of isothermal data requires finding or assuming a kinetic function that can properly fit the evolution of the reaction rate with time, so that the resulting parameters, i.e., the activation energy and pre-exponential factor, can be considered reliable. In the present work, we demonstrate using both simulated and experimental data that the kinetic analysis of a set of isothermal plots obtained at different temperatures, considering a single-step solid-state reaction, necessarily leads to the real activation energy, regardless the mathematical function selected for performing the kinetic analysis. This makes irrelevant the election of the kinetic function used to fit the experimental data and greatly facilitates the estimation of the activation energy for any single process.
Enero, 2015 | DOI: 10.1021/ef502269r
Reactividad de Sólidos
Limestone Calcination Nearby Equilibrium: Kinetics, CaO Crystal Structure, Sintering and Reactivity
Valverde, JM; Sanchez-Jimenez, PE; Perez-Maqueda, LAJournal of Physical Chemistry C, 119 (2015) 1523-1541
Show abstract ▽
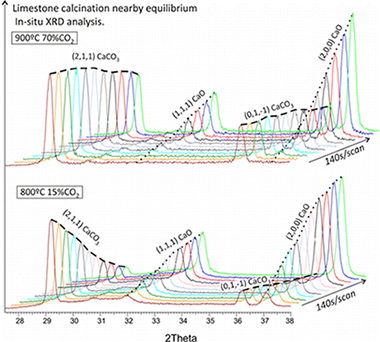
In this work, we analyze limestone calcination kinetics at environmental conditions involving a CO2 partial pressure P close to the equilibrium pressure Peq by means of in situ X-ray diffraction (XRD) and thermogravimetric (TG) analyses. In contrast with previous empirical observations carried out mostly at conditions far from equilibrium (P/Peq ≪ 1), our results show that the decarbonation rate decreases as the temperature in increased while P/Peq is kept constant, which is explained from a reaction mechanism including desorption of CO2 and the exothermic structural transformation from metastable CaO* nanocrystals to the stable CaO form. The crystal structure and sintering of nascent CaO during calcination has been investigated from in situ XRD analysis, physisorption analysis, and scanning electron microscopy (SEM), which shows that the ratio of the size of polycrystalline CaO grains to crystallite size increases linearly with the CO2 partial pressure in the calcination atmosphere. For high CO2 partial pressures, the size of CaO grains reaches a maximum value of around 1 μm, which leads to a residual surface area of about 1 m2/g, whereas in the limit P → 0 grain size and crystallite size (of the order of 10 nm) would coincide. Accordingly, sintering in the presence of CO2 would be triggered by the agglomeration of CaO crystals enhanced by CO2adsorption, which increases the surface energy. The carbonation reactivity of CaO resulting from calcination scales proportionally to its surface area and is not determined by a growth of the CaO exposed surface along a preferred crystallographic direction wherein carbonation would be unfavorable as suggested in recent works.
Enero, 2015 | DOI: 10.1021/jp508745u
2014
2014
Nanotecnología en Superficies y Plasma
Quinone-Rich Poly(dopamine) Magnetic Nanoparticles for Biosensor Applications
Martin, M; Orive, AG; Lorenzo-Luis, P; Creus, AH; Gonzalez-Mora, JL; Salazar, PChemPhysChem, 15 (2014) 3742-3752
Show abstract ▽
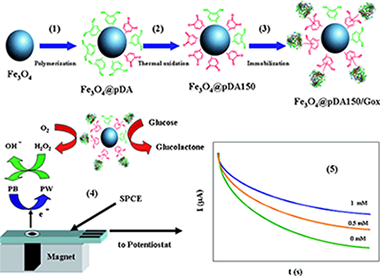
Novel core-shell quinone-rich poly(dopamine)–magnetic nanoparticles (MNPs) were prepared by using an in situ polymerization method. Catechol groups were oxidized to quinone by using a thermal treatment. MNPs were characterized by using X-ray diffraction, X-ray photoelectron spectroscopy, atomic force microscopy, magnetic force microscopy, UV/Vis, Fourier-transform infrared spectroscopy, and electrochemical techniques. The hybrid nanomaterial showed an average core diameter of 17 nm and a polymer-film thickness of 2 nm. The core-shell nanoparticles showed high reactivity and were used as solid supports for the covalent immobilization of glucose oxidase (Gox) through Schiff base formation and Michael addition. The amount of Gox immobilized onto the nanoparticle surface was almost twice that of the nonoxidized film. The resulting biofunctionalized MNPs were used to construct an amperometric biosensor for glucose. The enzyme biosensor has a sensitivity of 8.7 mA m−1 cm−2, a low limit of detection (0.02 mm), and high stability for 45 days. Finally, the biosensor was used to determine glucose in blood samples and was checked against a commercial glucometer.
Diciembre, 2014 | DOI: 10.1002/cphc.201402417
Propiedades mecánicas, modelización y caracterización de cerámicos avanzados
High temperature internal friction measurements of 3YTZP zirconia polycrystals. High temperature background and creep
Simas, P; Castillo-Rodriguez, M; No, ML; De-Bernardi, S; Gomez-Garcia, D; Dominguez-Rodriguez, A; Juan, JSJournal of the European Ceramic Society, 34 (2014) 3859-3863
Show abstract ▽

This work focuses on the high-temperature mechanic properties of a 3 mol% yttria zirconia polycrystals (3YTZP), fabricated by hot-pressureless sintering. Systematic measurements of mechanical loss as a function of temperature and frequency were performed. An analytical method, based on the generalized Maxwell rheological model, has been used to analyze the high temperature internal friction background (HTB). This method has been previously applied to intermetallic compounds but never to ceramics, except in a preliminary study performed on fine grain and nanocrystalline zirconia. The HTB increases exponentially and its analysis provides an apparent activation enthalpy which correlates well with that obtained from creep experiments. This fact shows on the one hand the plausibility of applying the generalized Maxwell model to ceramics, and on the other hand indicates the possibility of using mechanical spectroscopy as a complementary helpful technique to investigate the high temperature deformation mechanism of materials.
Diciembre, 2014 | DOI: 10.1016/j.jeurceramsoc.2014.05.016
- ‹ anterior
- 261 of 420
- siguiente ›














