Artículos SCI
2014
2014
Reactividad de Sólidos
Self-propagating combustion synthesis via an MSR process: An efficient and simple method to prepare (Ti, Zr, Hf)B2–Al2O3 powder nanocomposites
Sayagues, MJ; Aviles, MA; Cordoba, JM; Gotor, FJPowder Technology, 256 (2014) 244-250
Show abstract ▽
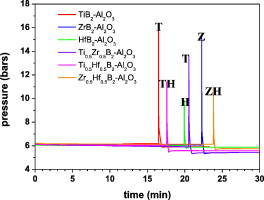
The synthesis of (Ti1 − xZrx)B2–Al2O3, (Ti1 − xHfx)B2–Al2O3 and (Zr1 − xHfx)B2–Al2O3 (x = 0, 0.5 and 1) powder nanocomposites via a mechanochemical method using TiO2, ZrO2, HfO2, HBO2 and Al as the raw materials was investigated. The formation of the nanocomposites proceeds via a mechanically-induced self-sustaining reaction (MSR) process that involves several simultaneous reactions. The aluminothermic reductions of the TMO2 and HBO2 produce Al2O3 and transition metal and boron elements, which in turn react to yield the diboride phase. The ignition of the complex combustion reaction occurred after a short milling time (15–30 min), instantly transforming most of the reactants into products. The sample composition was marked by the stoichiometry of the combustion reaction, and the resulting nanocomposites were analysed using XRD, ED, SEM, TEM and EDX techniques. The X-ray results confirmed the biphasic character of the prepared composite powder (TMB2 and Al2O3 structures); minor amounts of the Zr and Hf oxides were also observed. The achieved microstructure was characterised by the agglomeration of Al2O3 nanocrystallites and diboride crystals with a diffraction domain size ranging between 100 and 300 nm.
Abril, 2014 | DOI: 10.1016/j.powtec.2014.02.031
Reactividad de Sólidos
Nanosilica supported CaO: A regenerable and mechanically hard CO2 sorbent at Ca-looping conditions
Sanchez-Jimenez, PE; Perez-Maqueda, LA; Valverde, JMApplied Energy, 118 (2014) 92-99
Show abstract ▽
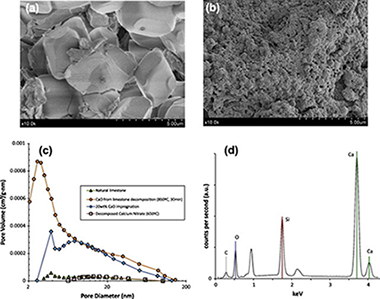
This work presents a CO2 sorbent that may be synthesized from low-cost and widely available materials following a simple method basically consisting of impregnation of a nanostructured silica support with a saturated solution of calcium nitrate. In a first impregnation stage, the use of a stoichiometric CaO/SiO2 ratio serves to produce a calcium silicate matrix after calcination. This calcium silicate matrix acts as a thermally stable and mechanically hard support for CaO deposited on it by further impregnation. The CaO-impregnated sorbent exhibits a stable CaO conversion at Ca-looping conditions whose value depends on the CaO wt% deposited on the calcium silicate matrix, which can be increased by successive reimpregnations. A 10 wt% CaO impregnated sorbent reaches a stable conversion above 0.6 whereas the stable conversion of a 30 wt% CaO impregnated sorbent is around 0.3, which is much larger than the residual conversion of CaO derived from natural limestone (between 0.07 and 0.08). Moreover, particle size distribution measurements of samples predispersed in a liquid and subjected to high energy ultrasonic waves indicate that the CaO-impregnated sorbent has a relatively high mechanical strength as compared to limestone derived CaO.
Abril, 2014 | DOI: 10.1016/j.apenergy.2013.12.024
Química de Superficies y Catálisis
Influence of the acid–base properties over NiSn/MgO–Al2O3 catalysts in the hydrogen production from glycerol steam reforming
Bobadilla, LF; Penkova, A; Romero-Sarria, F; Centeno, MA; Odriozola, JAInternational Journal of Hydrogen Energy, 39 (2014) 5704-5712
Show abstract ▽
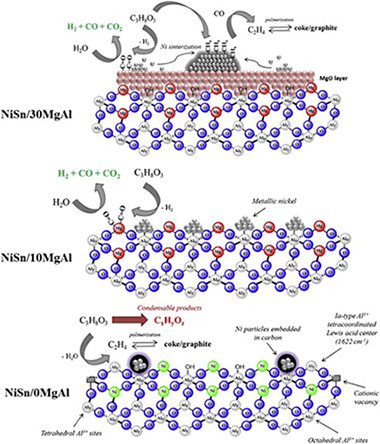
In this work we have investigated the hydrogen production from glycerol steam reforming. The effect of the acid-base properties was evaluated using four catalysts based in an alloy Ni-Sn as active phase supported over (Upsilon)-Al2O3 with different content in MgO, varying between 0 and 30 wt.% The incorporation of MgO results in the formation of MgAl2O4 spinel, which modifies the acid-base properties of the catalyst. Addition of MgO favored the glycerol conversion into gas, and the catalyst loaded with 10 wt.% MgO exhibited better catalytic performance and higher stability. A blank test with quartz was performed indicating that pyrolysis of glycerol takes place in the quartz.
Abril, 2014 | DOI: 10.1016/j.ijhydene.2014.01.136
Reactividad de Sólidos
Comparison of thermal behavior of natural and hot-washed sisal fibers based on their main components: Cellulose, xylan and lignin. TG-FTIR analysis of volatile products
Benitez-Guerrero, M; Lopez-Beceiro, J; Sanchez-Jimenez, PE; Pascual-Cosp, JThermochimica Acta, 581 (2014) 70-86
Show abstract ▽
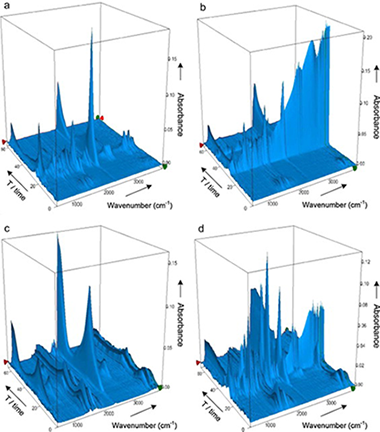
This paper presents in a comprehensive way the thermal behavior of natural and hot-washed sisal fibers, based on the fundamental components of lignocellulosic materials: cellulose, xylan and lignin. The research highlights the influence exerted on the thermal stability of sisal fibers by other constituents such as non-cellulosic polysaccharides (NCP) and mineral matter.
Thermal changes were investigated by thermal X-ray diffraction (TXRD), analyzing the crystallinity index (%Ic) of cellulosic samples, and by simultaneous thermogravimetric and differential thermal analysis coupled with Fourier-transformed infrared spectrometry (TG/DTA-FTIR), which allowed to examine the evolution of the main volatile compounds evolved during the degradation under inert and oxidizing atmospheres. The work demonstrates the potential of this technique to elucidate different steps during the thermal decomposition of sisal, providing extensible results to other lignocellulosic fibers, through the analysis of the evolution of CO2, CO, H2O, CH4, acetic acid, formic acid, methanol, formaldehyde and 2-butanone, and comparing it with the volatile products from pyrolysis of the biomass components. The hydroxyacetaldehyde detected during pyrolysis of sisal is indicative of an alternative route to that of levoglucosan, generated during cellulose pyrolysis.
Hot-washing at 75 °C mostly extracts non-cellulosic components of low decomposition temperature, and reduces the range of temperature in which sisal decomposition occurs, causing a retard in the pyrolysis stage and increasing TbNCP and TbCEL, temperatures at the maximum mass loss rate of non-cellulosic polysaccharides and cellulose decompositions, respectively. However, enriching sisal fibers in cellulose produces a decrease of TbCEL under an oxidizing atmosphere, and furthermore, a delay of the combustion process, displacing TbCOM to higher temperatures.
The results and findings of the paper would help further understanding of thermal processes where Agave fibers are involved, as the decomposition of their composites.
Abril, 2014 | DOI: 10.1016/j.tca.2014.02.013
Química de Superficies y Catálisis
Understanding the Role of the Cosolvent in the Zeolite Template Function of Imidazolium-Based Ionic Liquid
Ayala, R; Ivanova, S; Blanes, JMM; Romero-Sarria, F; Odriozola, JAJournal of Physical Chemistry B, 118 (2014) 3650–3660
Show abstract ▽
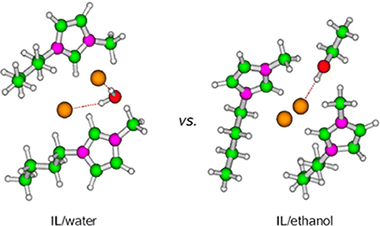
In this work, a study for understanding the role played by [ClBmim], [BF4Bmim], [PF6Bmim], and [CH3SO3Bmim] ionic liquids (ILs) in the synthesis of zeolites is presented. The use of [ClBmim] and [CH3SO3Bmim] ILs, as reported earlier [ Chem. Eur. J. 2013, 19, 2122] led to the formation of MFI or BEA type zeolites. Contrary, [BF4Bmim] and [PF6Bmim] ILs did not succeed in organizing the Si–Al network into a zeolite structure. To try to explain these results, a series of quantum mechanical calculations considering monomers ([XBmim]) and dimers ([XBmim]2) by themselves and plus cosolvent (water or ethanol) were carried out, where X ≡ Cl–, BF4–, PF6–, or CH3SO3–. Our attention was focused on the similarities and differences among the two types of cosolvents and the relation between the structure and the multiple factors defining the interactions among the ILs and the cosolvent. Although a specific pattern based on local structures explaining the different behavior of these ILs as a zeolite structuring template was not found, the calculated interaction energies involving the Cl– and CH3SO3– anions were very close and larger than those for BF4– and PF6– species. These differences in energy can be used as an argument to describe their different behavior as structure directing agents. Moreover, the topology of the cosolvent is also an ingredient to take into account for a proper understanding of the results.
Abril, 2014 | DOI: 10.1021/jp410260g
- ‹ anterior
- 280 of 420
- siguiente ›














