Artículos SCI
2011
2011
Materiales de Diseño para la Energía y Medioambiente
Structure and Chemical State of Octadecylamine Self-Assembled Monolayers on Mica
Benitez, JJ; San-Miguel, MA; Dominguez-Meister, S; Heredia-Guerrero, JA; Salmeron, MJournal of Physical Chemistry C, 115 (40) (2011) 19716-19723
Show abstract ▽
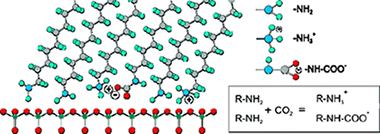
Structural and chemical data on n-octadecylamine self-assembled monolayers on mica (ODA/mica SAMs) have been obtained from ATR-FTIR and XPS spectroscopies. The analysis of the methylene modes concludes that alkylamine molecules are arranged in a rigid and well-ordered packing. Besides, the magnitude of the splitting of the methylene scissoring deflection is consistent with a molecular tilted configuration within the self-assembled layer, as already reported from topographic AFM data. Molecular dynamics simulations have supported this conclusion. XPS has revealed the presence of an important fraction of protonated amino groups (-NH3+) even in freshly prepared ODA/mica SAMs in air at RT. Two sources of protonation are proposed: (i) the acid-base reaction of (-NH2) end groups with the water adlayer on the surface of hydrophilic mica and (ii) the formation of an alkylamonium alkylcarbamate by a fast reaction with the atmospheric CO2 dissolved in such water adlayer. Though the water induced amino protonation is hypothetical, the presence of carbamate is univocally confirmed by ATR-FTIR. Upon extended contact with air (ripening) the conformational ordering in ODA/mica SAMs is slightly improved. Besides, further amino group protonation takes place with no additional carbamate formation. The process is described by a tentative mechanism in which protons are transferred from water molecules at the edges of SAMs islands to the inside. On the other side, carbamation is hindered by the steric effect of CO2 molecules trying to penetrate the close packed structure of octadecylamine molecules.
Octubre, 2011 | DOI: 10.1021/jp203871g
Materiales de Diseño para la Energía y Medioambiente
The effect of polymorphic structure on the structural and chemical stability of yttrium disilicates
Evgeny Galunin, Miquel Vidal and María D. AlbaAmerican Mineralogist, 96 (2011) 1512-1520
Show abstract ▽
Under pressure and temperature conditions like those found in deep geological repositories (DGRs), rare-earth cations may react with silicates to form rare-earth disilicates. This study establishes the stability range of yttrium disilicates in response to changes in pH. The α, β, γ, and δ polymorphs of Y2Si2O7 were synthesized by the sol-gel process at temperatures between 1100 and 1650 °C and subjected to pHstat leaching tests. By measuring the Y and Si leaching rates and monitoring the transformation of the crystalline and amorphous phases, we showed that yttrium disilicates were stable at pH > 5. At pH < 5, the pH stability sequence was consistent with the temperature-dependent stabilities of Y2Si2O7 phases, with the δ polymorph showing the lowest leaching rates. Because rare-earth compounds can be used as a proxy for analogous actinide hosts, the results of this study can be used to predict the performance of engineered barriers in DGR.
Octubre, 2011 | DOI: 10.2138/am.2011.3659
Materiales de Diseño para la Energía y Medioambiente
Evolution of Phases and Al–Si Distribution during Na-4-Mica Synthesis
María D. Alba, Miguel A. Castro, Moisés Naranjo, M. Mar Orta, Esperanza Pavón, and M. Carolina PazosJournal of Physical Chemistry C, 2011, 115 (41), pp 20084–20090
Show abstract ▽
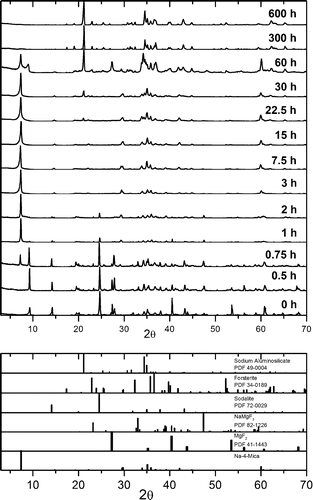
Na-4-mica, a highly charged fluorophlogopite, has recently attracted much attention because of its unique combination of high charge (four charges per unit cell) and its swelling and cation-exchange properties. The ability to improve the properties of this mica depends on gaining knowledge about its phase evolution during the calcination process. For the synthesis, the stoichiometric powder mixture (4SiO 2/2Al 2O 3/6MgF 2/8NaCl) was heated to 900 °C for 0-600 h. The obtained solids were characterized by X-ray fluorescence (XRF); X-ray diffraction (XRD); scanning electron microscopy/energy-dispersive X-ray (SEM/EDX) analysis; 29Si, 27Al, and 23Na magic-angle-spinning MAS NMR spectroscopy; and thermogravimetric/differential thermal analysis (TG/DTA). The results showed that the precursors are rapidly (t < 3 h) transformed into sodalite, Al 6Na 8(SiO 4) 6Cl 2, and a 2:1 phyllosilicate. For t = 7.5 h, the amount of 2:1 phyllosilicate increased as a result of the decomposition of sodalite, with a progressive incorporation of aluminum in the 2:1 phyllosilicate being observed. For t = 7.5 h, synthesis of Na-4-mica was considered to be complete, as the material remained essentially unaltered for the next 15 h. For t = 30 h, the mica started to decompose, and for very long reaction times (t ≥ 300 h), only forsterite and a carnegierite phase were present.
Octubre, 2011 | DOI: 10.1021/jp207408h
Materiales Nanoestructurados y Microestructura
Magnetic and fluorescent core-shell nanoparticles for ratiometric pH sensing
Lapresta-Fernández, A., Doussineau, T., Dutz, S., Steiniger, F., Moro, A.J., Mohr, G.J.Nanotechnology, 22 (2011), Article number 415501
Show abstract ▽
This paper describes the preparation of nanoparticles composed of a magnetic core surrounded by two successive silica shells embedding two fluorophores, showing uniform nanoparticle size (50-60nm in diameter) and shape, which allow ratiometric pH measurements in the pH range 5-8. Uncoated iron oxide magnetic nanoparticles (∼10nm in diameter) were formed by the coprecipitation reaction of ferrous and ferric salts. Then, they were added to a water-in-oil microemulsion where the hydrophilic silica shells were obtained through hydrolysis and condensation of tetraethoxyorthosilicate together with the corresponding silylated dye derivatives - a sulforhodamine was embedded in the inner silica shell and used as the reference dye while a pH-sensitive fluorescein was incorporated in the outer shell as the pH indicator. The magnetic nanoparticles were characterized using vibrating sample magnetometry, dynamic light scattering, transmission electron microscopy, x-ray diffraction and Fourier transform infrared spectroscopy. The relationship between the analytical parameter, that is, the ratio of fluorescence between the sensing and reference dyes versus the pH was adjusted to a sigmoidal fit using a Boltzmann type equation giving an apparent pKa value of 6.8. The fluorescence intensity of the reference dye did not change significantly (∼3.0%) on modifying the pH of the nanoparticle dispersion. Finally, the proposed method was statistically validated against a reference procedure using samples of water and physiological buffer with 2% of horse serum, indicating that there are no significant statistical differences at a 95% confidence level.
Octubre, 2011 | DOI: 10.1088/0957-4484/22/41/415501
Nanotecnología en Superficies y Plasma
Enhanced gas sensing performance of TiO2 functionalized magneto-optical SPR sensors
M.G. Manera, G. Montagna, E. Ferreiro-Vila, L. González-García, J.R. Sánchez-Valencia, A.R. González-Elipe, A.Cebollada, J.M. Garcia-Martin, A. Garcia-Martin, G. Armelles and R. RellaJournal of Materials Chemistry, 21 (2011) 16049-16056
Show abstract ▽
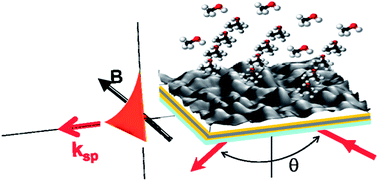
Porous TiO2 thin films deposited by glancing angle deposition are used as sensing layers to monitor their sensing capabilities towards Volatile Organic Compounds both in a standard Surface Plasmon Resonance (SPR) sensor and in Magneto-Optical Surface Plasmon Resonance (MO-SPR) configuration in order to compare their sensing performances. Here our results on the enhanced sensing capability of these TiO2 functionalized MO-SPR sensors with Au/Co/Au transducers with respect to traditional SPR gas sensors are presented.
Octubre, 2011 | DOI: 10.1039/c1jm11937k
- ‹ anterior
- 347 of 422
- siguiente ›














