Artículos SCI
2011
2011
Nanotecnología en Superficies y Plasma
Theoretical and experimental characterization of TiO2 thin films deposited at oblique angles
Álvarez, R., González-García, L., Romero-Gómez, P., Rico, V., Cotrino, J., Gonzalez-Elipe, A.R., Palmero, A.Journal of Physics D: Applied Physics, 44 (2011) Article number 385302
Show abstract ▽
The microstructural features of amorphous TiO2 thin films grown by the electron beam physical vapour deposition technique at oblique angles have been experimentally and theoretically studied. The microstructural features of the deposited films were characterized by considering both the column tilt angle and the increase in the column thickness with height. A Monte Carlo model of film growth has been developed that takes into account surface shadowing, short-range interaction between the deposition species and the film surface, as well as the angular broadening of the deposition flux when arriving at the substrate. The good match between simulations and experimental results indicates the importance of these factors in the growth and microstructural development of thin films deposited at oblique angles.
Septiembre, 2011 | DOI: 10.1088/0022-3727/44/38/385302
Materiales de Diseño para la Energía y Medioambiente
Structural elucidation of Β-(Y,Sc)2Si2O 7: Combined use of 89Y MAS NMR and powder diffraction
Allix, M., Alba, M.D., Florian, P., Fernandez-Carrion, A.J., Suchomel, M.R., Escudero, A., Suard, E., Becerro, A.I.Journal of Applied Crystallography, 44 (2011) 846-852
Show abstract ▽
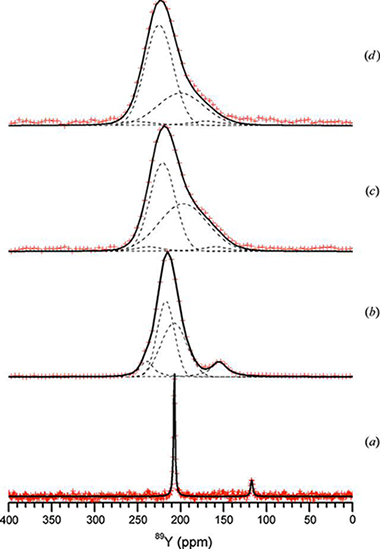
Although the structures of pure Sc2Si2O7 and Β-Y2Si 2O7 have been described in the literature using the C2/m space group, 29Si magic angle spinning (MAS) NMR measurements of the intermediate members of the Sc2Si2O7-Β- Y2Si2O7 system indicate a lowering of the symmetry to the C2 space group. Indeed, these compositions exhibit a unique Si crystallographic site and an Si-O-Si angle lower than 180°, incompatible with the C2/m space group. C2 is the only possible alternative. Space group Cm can be discarded with regard to its two different Si sites per unit cell. Moreover, 89Y MAS NMR data have revealed the existence of two different Y sites in the structure of the intermediate members of the Sc 2Si2O7-Β-Y2Si2O 7 system, confirming the lowering of the symmetry to the C2 space group. The viability of the C2 model has therefore been tested and confirmed by refinement of synchrotron and neutron powder diffraction data for the different members of the system. The structural evolutions across the Sc 2Si2O7-Β-Y2Si2O 7 system are discussed.
Agosto, 2011 | DOI: 10.1107/S0021889811021303
Nanotecnología en Superficies y Plasma
Aspects of heterogeneous enantioselective catalysis by metals
Kyriakou, G., Beaumont, S.K., Lambert, R.M.Langmuir, 27 (2011) 9687-9695
Show abstract ▽
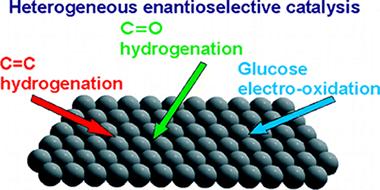
Some aspects of metal-catalyzed heterogeneous enantioselective reactions are reviewed with specific reference to four different systems where the phenomena that control enantioselection appear to be very different. In the case of glucose electro-oxidation, it is clear that any intrinsic chirality present at the metal surface plays a vital role. With α-keto hydrogenation, achiral surfaces modified by the adsorption of chiral agents become effective enantioselective catalysts and the formation of extended arrays of chiral species appears not to be of importance: instead a 1:1 docking interaction controlled by hydrogen bonding between the adsorbed chiral modifier and the prochiral reactant determines the outcome. Hydrogen bonding also plays a central role in β-ketoester hydrogenation, but here fundamental studies indicate that the formation of ordered arrays involving the reactant and chiral ligand is of importance. Asymmetric C=C hydrogenation, though relatively little studied, has the potential for major impact in synthetic organic chemistry both on the laboratory scale and in the manufacture of fine chemicals and pharmaceuticals. The structural attributes that determine whether a given chiral ligand is effective have been identified; the ability to form strong covalent bonds with the metal surface while also resisting hydrogenation and displacement by the strongly adsorbing reactant under reaction conditions is an essential necessary condition. Beyond this, ligand rigidity in the vicinity of the chirality center coupled with resistance to SAM formation is a critically important factor whose absence results in racemic chemistry.
Agosto, 2011 | DOI: 10.1021/la200009w
Materiales de Diseño para la Energía y Medioambiente
Formation of organo-highly charged mica
Alba, M.D. , Castro, M.A., Orta, M.M., Pavón, E., Pazos, M.C., Valencia Rios, J.S.Langmuir, 16 (2011) 9711-9718
Show abstract ▽
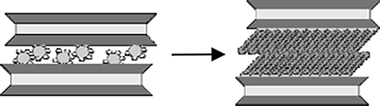
The interlayer space of the highly charged synthetic Na-Mica-4 can be modified by ion-exchange reactions involving the exchange of inorganic Na + cations by surfactant molecules, which results in the formation of an organophilic interlayer space. The swelling and structural properties of this highly charged mica upon intercalation with n-alkylammonium (RNH 3) + cations with varying alkyl chain lengths (R = C12, C14, C16, and C18) have been reported. The stability, fine structure, and evolution of gaseous species from alkylammonium Mica-4 are investigated in detail by conventional thermogravimetric analysis (TGA), Fourier transform infrared spectroscopy (FTIR), in situ X-ray diffraction (XRD), and solid-state nuclear magnetic resonance (MAS NMR) techniques. The results clearly show the total adsorption of n-alkylammonium cations in the interlayer space which expands as needed to accommodate intercalated surfactants. The surfactant packing is quite ordered at room temperature, mainly involving a paraffin-type bilayer with an all-trans conformation, in agreement with the high density of the organic compounds in the interlayer space. At temperatures above 160 °C, the surfactant molecules undergo a transformation that leads to a liquid-like conformation, which results in a more disordered phase and expansion of the interlayer space.
Agosto, 2011 | DOI: 10.1021/la200942u
Materiales Nanoestructurados y Microestructura
Boron Compounds as Stabilizers of a Complex Microstructure in a Co-B-based Catalyst for NaBH4 Hydrolysis
Arzac, G.M., Rojas, T.C., Fernández, A.ChemCatChem, 3 (2011) 1305-1313
Show abstract ▽
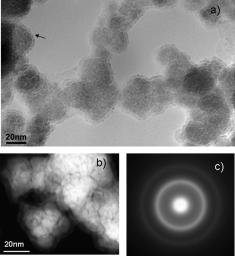
Co-B-based materials are widely used as catalysts for hydrogen generation through sodium borohydride self-decomposition. In the mid 1990s, the aqueous and organic chemistry involved in Co-B synthesis and handling was studied. Nevertheless, the exact microstructure of these catalysts has remained unsolved. Herein we present an exhaustive study which shows a new and complete microstructural view of a Co-B-based material together with the chemistry of the cobalt and boron involved. By using nanoscale-resolution microscopy and spectroscopy techniques, we have elucidated the role of boron compounds as stabilizers in a complex microstructure, which also explains its high catalytic performance and long-term stability. The catalyst is proposed to be made up of 1-3nm hcp Co0 nanoparticles embedded in amorphous CoxB (x=1, 2, 3), CoxOy, Co(BO2)2, and B2O3 phases alternatively or all together. All of these amorphous phases protect the nanocrystalline metallic core from growth and oxidation.
Agosto, 2011 | DOI: 10.1002/cctc.201100101
- ‹ anterior
- 349 of 422
- siguiente ›














