Artículos SCI
2010
2010
Reactividad de Sólidos
Mechanochemical synthesis of vanadium nitride
Roldan, MA; Lopez-Flores, V; Alcala, MD; Ortega, A; Real, CJournal of the European Ceramic Society, 30 (2010) 2099-2107
Show abstract ▽
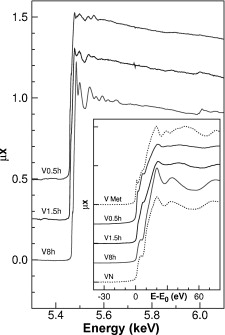
Vanadium nitride (VN) has been prepared by mechanosynthesis from vanadium metal under a pressurized nitrogen atmosphere in a short milling time. The characterization of the final product by X-ray diffraction, scanning electron microscopy, electron energy loss (EELS), and X-ray absorption spectroscopy (XAS) is presented. The final product, VN with 96% of purity, is obtained at room temperature with nanometric particle size and a very high microhardness after sintering. A relationship between microstructure and microhardness as well as a comparison between the VN obtained mechanical and thermal method is also presented.
Agosto, 2010 | DOI: 10.1016/j.jeurceramsoc.2010.04.008
Study of the Dehydroxylation-Rehydroxylation of Pyrophyllite
Perez-Rodriguez, JL; Duran, A; Sanchez-Jimenez, PE; Franquelo, ML; Perejon, A; Pascual-Cosp, J; Perez-Maqueda, LAJournal of the American Ceramic Society, 93 (2010) 2392-2398
Show abstract ▽
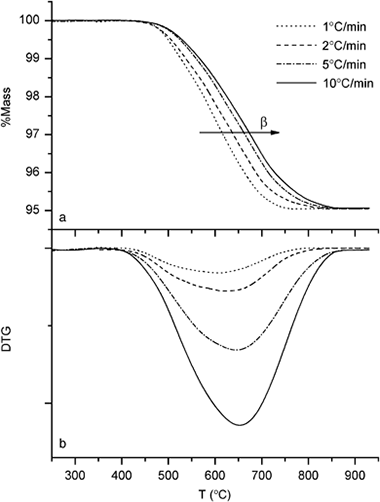
Pyrophyllite is a raw material of significant interest due to its large number of applications. Most of these applications require a thermal transformation of pyrophyllite; this thermal transformation implies the release of structural OH groups and the formation of new phases. In this paper, we report on the dehydroxylation of pyrophyllite and the reversibility of the process. A value of 224 +/- 16 kJ/mol for the dehydroxylation of pyrophyllite was obtained. In addition, it was observed that the partially or totally dehydroxylated pyrophyllite suffered a partial reversible rehydroxylation when cooled to room temperature. This rehydroxylation was substantiated by thermogravimetric measurements, while infrared spectroscopic studies showed that, during the rehydroxylation, the intensity of the OH band at 3675 cm-1 increased as two new bands at 3690 and 3702 cm-1 appeared. This rehydroxylation process was heavily influenced by the particle size of the pyrophyllite. Thus, smaller particles (< 1 mu m) showed a larger rehydroxylation percentage (about 12%), while the larger ones (20-40 mu m) showed a smaller percentage (about 1.6%). The extent of rehydroxylation also depended on the dehydroxylation temperature and reached a maximum value at 750 degrees C.
Agosto, 2010 | DOI: 10.1111/j.1551-2916.2010.03750.x
Materiales y Procesos Catalíticos de Interés Ambiental y Energético
Study of nanostructured Ni/CeO2 catalysts prepared by combustion synthesis in dry reforming of methane
Gonzalez-Delacruz, VM; Ternero, F; Pereniguez, R; Caballero, A; Holgado, JPApplied Catalysis A-General, 384 (2010) 1-9
Show abstract ▽
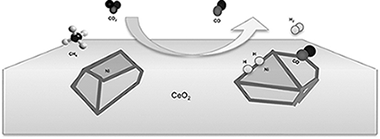
This work reports the study of several catalysts of Ni-CeO2 active for dry methane reforming process (CH4 + CO2 -> 2CO + 2H(2)). The use of Ni as active phase is highly preferred, due to its availability, high activity and low cost, although its main lack is the coke formation on the surface of Ni metal particles, resulting in a severe deactivation. Here we report a new synthesis method that allows a simple, effective and fast way to prepare Ni-CeO2 catalysts, in a wide range of metallic loadings, resulting in all the cases in well-formed NiO crystallites with sizes in the range of 12-18 nm. The use of CeO2 as a support has been based on its massive use in TWC catalysts formulations, where it is recognized to activate CH4 and lower hydrocarbon dissociation. Moreover, CeO2 has been reported to have an intrinsic activity in the CH4 reforming reaction. Besides the metallic loading, several factors that control the preparation method of the catalyst have been varied, in order to optimize their performance. Most of the catalysts prepared show activity and selectivity values close to thermodynamic ones, maintaining a good stability on long periods of time and severe conditions. Nevertheless, formation of some carbon nano-fibers has been observed, which could result in a drawback for their application at large scale.
Agosto, 2010 | DOI: 10.1016/j.apcata.2010.05.027
Reactividad de Sólidos
Kinetic model for thermal dehydrochlorination of poly(vinyl chloride)
Sanchez-Jimenez, PE; Perejon, A; Criado, JM; Dianez, MJ; Perez-Maqueda, LAPolymer, 51 (2010) 3998-4007
Show abstract ▽
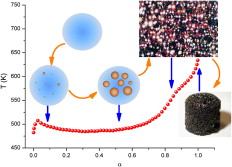
In this paper, a novel method for calculating degradation kinetics is presented. The method has been applied to the thermal dehydrochlorination of two different samples of PVC. It has been observed that this dehydrochlorination is complex and involves two different processes. A model that accounts for the entire dehydrochlorination is proposed. This model involves nucleation and growth and diffusion controlled mechanisms. The kinetic parameters are obtained from linear heating rate, isothermal and sample controlled thermal analysis experiments. Kinetic results obtained from the macroscopic thermal analysis measurements demonstrate the correlation between the kinetics of the thermal dehydrochlorination of PVC and the structure of this macromolecule.
Agosto, 2010 | DOI: 10.1016/j.polymer.2010.06.020
Reactividad de Sólidos
Generalized Kinetic Master Plots for the Thermal Degradation of Polymers Following a Random Scission Mechanism
Sanchez-Jimenez, PE; Perez-Maqueda, LA; Perejon, A; Criado, JMJournal of Physical Chemistry A, 114 (2010) 7868-7876
Show abstract ▽
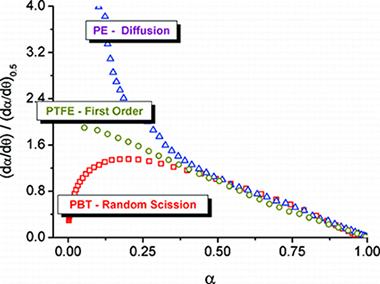
In this paper, the f(alpha) conversion functions for random scission mechanisms have been proposed to allow for the construction of generalized master plots suitable for these kinds of mechanisms. The master plots have been validated by their application to simulated data and to the thermal degradation of poly(butylene terephthalate), polyethylene, and poly(tetrafluoroethylene).
Agosto, 2010 | DOI: 10.1021/jp103171h
- ‹ anterior
- 373 of 422
- siguiente ›














