Artículos SCI
2009
2009
Materiales de Diseño para la Energía y Medioambiente
Chemical Reactions in 2D: Self-Assembly and Self-Esterification of 9(10),16-Dihydroxypalmitic Acid on Mica Surface
Heredia-Guerrero, JA; San-Miguel, MA; Sansom, MSP; Heredia, A; Benitez, JJLangmuir, 25 (2009) 6869-6874
Show abstract ▽
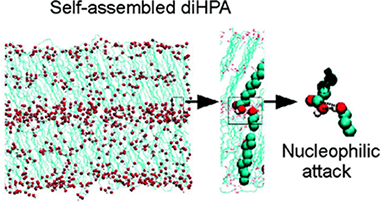
9(10),16-Dihydroxypalmitic acid (diHPA) is a particularly interesting polyhydroxylated fatty acid (1) because it is the main monomer of cutin, the most abundant biopolyester in nature, and (2) because the presence of a terminal and a secondary hydroxyl group in midchain positions provides an excellent model to study their intermolecular interactions in a confined phase such as self-assembled layers. In this study we have combined atomic force microscopy (AFM), X-ray photoelectron spectroscopy (XPS), attenuated total reflection Fourier transform infrared (ATR-FT-IR) spectroscopy, as well as molecular dynamics (MD) simulations to conclude that the self-assembling of diHPA molecules on mica is a layer by layer process following a Brunauer−Emmett−Teller (BET) type isotherm and with the first layer growing much faster than the rest. Interactions between secondary hydroxyls reinforce the cohesive energy of the monolayer, while the presence of the terminal hydroxyl group is necessary to trigger the multilayered growth. Besides, XPS and ATR-FT-IR spectroscopies clearly indicate that spontaneous self-esterification occurs upon self-assembling. The esterification reaction is a prerequisite to propose a self-assembly route for the biosynthesis of cutin in nature. Molecular dynamics simulations have shown that internal molecular reorganization within the self-assembled layers provides the appropriate intermolecular orientation to facilitate the nucleophilic attack and the release of a water molecule required by the esterification reaction.
Junio, 2009 | DOI: 10.1021/la9001412
Materiales Nanoestructurados y Microestructura
Thermal Evolution of WC/C Nanostructured Coatings by Raman and In Situ XRD Analysis
El Mrabet, S; Abad, MD; Lopez-Cartes, C; Martinez-Martinez, D; Sanchez-Lopez, JCPlasma Processes and Polymers, 6 (2009) S444-S449
Show abstract ▽
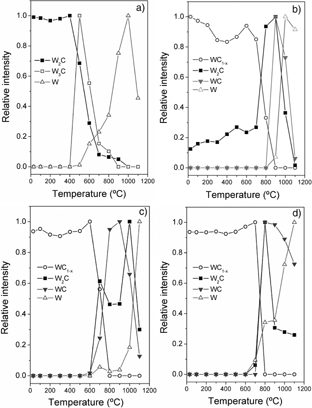
In this work, a series of WC/C nanostructured films were deposited on silicon substrates by changing the ratio of sputtering power applied to graphite and WC magnetron sources (PC/PWC: 0, 0.1, 0.5, 1). The thermal stability of WC/C coatings was followed in situ by means of X-ray diffraction measurements up to 1 100 °C in vacuum (10−1 Pa). Initially, the film microstructure is composed of nanocrystalline WC1−x and W2C phases. As the PC/PWC ratio increases the crystallinity decreases, and WC1−x becomes the predominant phase from PC/PWC = 0.1. The results show that the structural evolution with temperature of all studied layers depends essentially on their initial phase and chemical composition (determined by the synthesis conditions: ratio PC/PWC). The coating deposited at PC/PWC = 0 reveals a transformation of W2C phase into W and W3C phases at 400 °C. However, the samples with PC/PWC greater than 0 exhibits an improved thermal stability up to 600–700 °C where the WC1−x begins to transform into W2C and WC phases. At 900 °C, WC is the predominant phase, especially for those coatings prepared with higher ratios. Further annealing above 1 000 °C yields W as the foremost phase. The thermal behaviour was later studied by means of Raman spectroscopy measurements at certain temperatures where the main changes in phase composition were observed. Particularly, a fitting analysis was carried out on the D and G bands typical of disordered and amorphous carbon. The changes induced during heating are discussed in terms of the positions of D and G lines, and full width at half maximum (FWHM).
Junio, 2009 | DOI: 10.1002/ppap.200931004
Materiales de Diseño para la Energía y Medioambiente
Application of 29Si and 27Al MAS NMR Spectroscopy to the Study of the Reaction Mechanism of Kaolinite to Illite/Muscovite
Mantovani, M; Escudero, A; Becerro, AIClays and Clay Minerals, 57 (2009) 302-310
Show abstract ▽
Understanding the mechanisms for illitization of clay minerals has important applications in reconstructing geologic histories and determining the origins of physical and chemical characteristics of buried sediments. While many studies have been carried out on this topic, few have focused on the mechanism of illite formation from kaolinite. The purpose of this study was to investigate more deeply the illitization of kaolinite in KOH solution at a high solid/liquid ratio (1000 mg/mL). X-ray diffraction (XRD) and infrared spectroscopy were used to follow the formation of new crystalline phases and the composition of the octahedral sheet, while the transformation of the Si and Al local environments was analyzed by 29Si and 27Al magic angle spinning nuclear magnetic resonance spectroscopy (MAS NMR). The results revealed that the first reaction stage consists of the diffusion of Al from the octahedral to the tetrahedral sheet of the kaolinite TO layers, giving rise to the precursors of the illite/muscovite nuclei. Combination of XRD with 27Al MAS NMR measurements indicated that a minimum amount of tetrahedral Al is required in the original TO layer before condensation of a second tetrahedral sheet occurs to complete the formation of the illite/muscovite TOT layers.
Junio, 2009 | DOI: 10.1346/CCMN.2009.0570303
Química de Superficies y Catálisis
Determination of nitrogen partitioning coefficients in superduplex stainless steels by NRA using a nuclear microprobe
Munoz, C; Morilla, Y; Lopez, JG; Paul, A; Odriozola, JANuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 267 (2009) 2208-2211
Show abstract ▽

Superduplex stainless steels (SDSSs) combine the good mechanical behavior and the high corrosion resistance of the ferrite (α-Fe) and austenite (γ-Fe) phases. The SDSSs properties depend strongly on the partitioning of the elements that form the alloy. The ferrite is generally enriched in P, Si, Cr and Mo while the content of Ni, Mn, Cu and N in the austenite phase is higher. Nitrogen is known to be a strong austenite stabilizer and its presence increases the strength and the pitting corrosion resistance of the stainless steels. While the global nitrogen content in SDSSs can be readily determined using elemental analyzers, it cannot be measured at a microscopic scale.
In this work, the nuclear microprobe of the Centro Nacional de Aceleradores (Sevilla) was used to obtain the quantitative distribution of nitrogen in SDSSs. A deuteron beam of 1.8 MeV was employed to determine the overall elemental concentration of the matrix by deuteron-induced X-ray emission, whereas the nitrogen partitioning coefficients were obtained by using the 14N(d, α0)12C nuclear reaction. Mappings of this element show that the nitrogen ratio between the ferrite and austenite phases ranges from 0.3 to 0.6 in the analyzed samples.
Junio, 2009 | DOI: 10.1016/j.nimb.2009.03.093
Química de Superficies y Catálisis
Fibrous MnO2 Nanoparticles with (2 × 2) Tunnel Structures. Catalytic Activity in the Total Oxidation of Volatile Organic Compounds
Dominguez, MI; Navarro, P; Romero-Sarria, F; Frias, D; Cruz, SA; Delgado, JJ; Centeno, MA; Montes, M; Odriozola, JAJournal of Nanoscience and Nanotechnology, 9 (2009) 3837-3842
Show abstract ▽
Manganese oxides having 2 × 2 tunnel structures (cryptomelanes) have been synthesized by a milling method in order to test their efficiency as catalysts for the abatement of volatile organic compounds, using toluene as probe molecule. These materials present excellent textural properties, arising from the nanofiber morphology and were active for toluene total oxidation. DRIFTS of the adsorbed phase allow proposing the role of lattice oxygen in the catalytic reaction.
Junio, 2009 | DOI: 10.1166/jnn.2009.NS76
- ‹ anterior
- 397 of 422
- siguiente ›














